Pathogenetic mechanisms of focal cortical dysplasia
- PMID: 24861491
- PMCID: PMC4107035
- DOI: 10.1111/epi.12650
Pathogenetic mechanisms of focal cortical dysplasia
Abstract
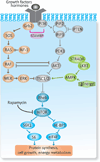
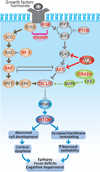
Focal cortical dysplasias (FCDs) constitute a prevalent cause of intractable epilepsy in children, and is one of the leading conditions requiring epilepsy surgery. Despite recent advances in the cellular and molecular biology of these conditions, the pathogenetic mechanisms of FCDs remain largely unknown. The purpose if this work is to review the molecular underpinnings of FCDs and to highlight potential therapeutic targets. A systematic review of the literature regarding the histologic, molecular, and electrophysiologic aspects of FCDs was conducted. Disruption of the mammalian target of rapamycin (mTOR) signaling comprises a common pathway underlying the structural and electrical disturbances of some FCDs. Other mechanisms such as viral infections, prematurity, head trauma, and brain tumors are also posited. mTOR inhibitors (i.e., rapamycin) have shown positive results on seizure management in animal models and in a small cohort of patients with FCD. Encouraging progress has been achieved on the molecular and electrophysiologic basis of constitutive cells in the dysplastic tissue. Despite the promising results of mTOR inhibitors, large-scale randomized trials are in need to evaluate their efficacy and side effects, along with additional mechanistic studies for the development of novel, molecular-based diagnostic and therapeutic approaches.
Keywords: Focal cortical dysplasia; Giant cell; Mammalian target of rapamycin; PMSE syndrome; mTOR.
Wiley Periodicals, Inc. © 2014 International League Against Epilepsy.
Conflict of interest statement
None to declare.
Figures

Similar articles
-
Focal malformations of cortical development: new vistas for molecular pathogenesis.Neuroscience. 2013 Nov 12;252:262-76. doi: 10.1016/j.neuroscience.2013.07.037. Epub 2013 Jul 25. Neuroscience. 2013. PMID: 23892008 Review.
-
Deep histopathology genotype-phenotype analysis of focal cortical dysplasia type II differentiates between the GATOR1-altered autophagocytic subtype IIa and MTOR-altered migration deficient subtype IIb.Acta Neuropathol Commun. 2023 Nov 9;11(1):179. doi: 10.1186/s40478-023-01675-x. Acta Neuropathol Commun. 2023. PMID: 37946310 Free PMC article.
-
Alterations in dopaminergic innervation and receptors in focal cortical dysplasia.Brain. 2025 Aug 1;148(8):2899-2911. doi: 10.1093/brain/awaf080. Brain. 2025. PMID: 40235315 Free PMC article.
-
Idiopathic (Genetic) Generalized Epilepsy.2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31536218 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
Cited by
-
Genetic animal models of malformations of cortical development and epilepsy.J Neurosci Methods. 2016 Feb 15;260:73-82. doi: 10.1016/j.jneumeth.2015.04.007. Epub 2015 Apr 21. J Neurosci Methods. 2016. PMID: 25911067 Free PMC article. Review.
-
Dysregulation of NEUROG2 plays a key role in focal cortical dysplasia.Ann Neurol. 2018 Mar;83(3):623-635. doi: 10.1002/ana.25187. Epub 2018 Mar 10. Ann Neurol. 2018. PMID: 29461643 Free PMC article.
-
Inhibition of mTOR Pathway by Rapamycin Decreases P-glycoprotein Expression and Spontaneous Seizures in Pharmacoresistant Epilepsy.J Mol Neurosci. 2017 Apr;61(4):553-562. doi: 10.1007/s12031-017-0897-x. Epub 2017 Feb 22. J Mol Neurosci. 2017. PMID: 28229367
-
Establishment of a Regional Interdisciplinary Medical System for Managing Patients with Tuberous Sclerosis Complex (TSC).Sci Rep. 2018 Nov 13;8(1):16747. doi: 10.1038/s41598-018-35168-y. Sci Rep. 2018. PMID: 30425292 Free PMC article.
-
Clinical and Surgical Approach for Cerebral Cortical Dysplasia.Adv Tech Stand Neurosurg. 2023;48:327-354. doi: 10.1007/978-3-031-36785-4_12. Adv Tech Stand Neurosurg. 2023. PMID: 37770690
References
-
- Ak H, Ay B, Tanriverdi T, et al. Expression and cellular distribution of multidrug resistance-related proteins in patients with focal cortical dysplasia. Seizure. 2007;16:493–503. - PubMed
-
- Alonso-Nanclares L, Garbelli R, Sola RG, et al. Microanatomy of the dysplastic neocortex from epileptic patients. Brain. 2005;128:158–173. - PubMed
-
- Andre VM, Flores-Hernandez J, Cepeda C, et al. NMDA receptor alterations in neurons from pediatric cortical dysplasia tissue. Cereb Cortex. 2004;14:634–646. - PubMed
-
- Arai Y, Edwards V, Becker LE. A comparison of cell phenotypes in hemimegalencephaly and tuberous sclerosis. Acta Neuropathol. 1999;98:407–413. - PubMed
-
- Aronica E, Boer K, Baybis M, et al. Co-expression of cyclin D1 and phosphorylated ribosomal S6 proteins in hemimegalencephaly. Acta Neuropathol. 2007;114:287–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

