Mitogen-activated protein kinase (MAPK) phosphatase-3 (MKP-3) displays a p-JNK-MAPK substrate preference in astrocytes in vitro
- PMID: 24861519
- PMCID: PMC8365871
- DOI: 10.1016/j.neulet.2014.05.039
Mitogen-activated protein kinase (MAPK) phosphatase-3 (MKP-3) displays a p-JNK-MAPK substrate preference in astrocytes in vitro
Abstract
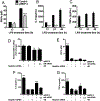
Mitogen-activated protein kinases (MAPKs) play critical roles in the central nervous system immune responses through glial function, which are regulated with relative selectivity (or preference) by MAPK phosphatases (MKP). Phosphorylated extracellular signal-regulated protein kinase (p-ERK) is preferentially dephosphorylated by MKP-3, which display little activity over p-p38 and p-c-Jun NH2-terminal kinases (p-JNK). It has been proposed that these substrate preferences may vary depending on tissue or functional cellular processes. Since astrocytes display a prominent activity of JNK>ERK under stressed or reactive phenotype, we hypothesize that MKP-3 possess a similar or differential substrate preference in astrocytes for JNK and ERK (ERK=JNK or JNK>ERK). We generated transient expression of MKP-3 by transfecting a specific cDNA in primary rat neonatal brain cortex astrocytes. Cells were stimulated with lipopolysaccharide (LPS), and MAPKs and downstream pro-inflammatory products were measured by Western blot and ELISA analyses. MKP-3 expression in primary astrocytes reduced LPS-induced p-ERK and p-p38 by ∼50%, and p-JNK by ∼75%, and moderately reduced nitrite oxide (NO), while completely blocked Interleukin (IL)-6 and tumor necrosis factor alpha (TNFα). We confirmed MKP-3 specific activity by developing a BV-2 microglia cell line stably overexpressing MKP-3 and using a specific siRNA against MKP-3. Our data demonstrate MKP-3 has differential substrate preference in astrocytes compared to other cells types, since it preferentially dephosphorylated p-JNK over p-ERK. Our results indicate also that astrocytic immune functions can be modulated by MKP-3 induction, a strategy that could be beneficial in neurological conditions in which astrocytes play a pathophysiological role, i.e. persistent pain.
Keywords: Astrocytes; Cytokines; Glia; MKP-3; Microglia; Phosphatase.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Competing interests
The authors declare that they have no competing interests.
Figures

References
-
- Anand P, Shenoy R, Palmer JE, Baines AJ, Lai RY, Robertson J, Bird N, Ostenfeld T, Chizh BA, Clinical trial of the p38 MAP kinase inhibitor dilmapi-mod in neuropathic pain following nerve injury, Eur. J. Pain 15 (2011) 1040–1048. - PubMed
-
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA, Intrathecal anti-IL-6 antibody and IgG attenuates peripheral nerve injury-induced mechanical allodynia in the rat: possible immune modulation in neuropathic pain, Brain Res. 879 (2000) 216–225. - PubMed
-
- Ducruet AP, Vogt A, Wipf P, Lazo JS, Dual specificity protein phosphatases: therapeutic targets for cancer and Alzheimer’s disease, Annu. Rev. Pharmacol. Toxicol 45 (2005) 725–750. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

