Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer
- PMID: 24861561
- PMCID: PMC4111281
- DOI: 10.1093/bja/aeu090
Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer
Abstract
Background: Morphine stimulates angiogenesis and cancer progression in mice. We investigated whether morphine influences tumour onset, development, and animal model survival, and whether µ-opioid receptor (MOR), lymphangiogenesis, mast cell activation, and substance P (SP) are associated with the tumour-promoting effects of morphine.
Methods: Transgenic mice with a rat C3(1) simian virus 40 large tumour antigen fusion gene which demonstrate the developmental spectrum of human infiltrating ductal breast carcinoma were used. Mice were treated at different ages with clinically relevant doses of morphine or phosphate-buffered saline to determine the effect on tumour development and progression, and on mouse survival. Tumours were analysed for MOR, angiogenesis, lymphangiogenesis, SP, and mast cell activation by immunofluorescent- or laser scanning confocal-microscopy. Cytokine and SP levels were determined by enzyme-linked immunosorbent assay.
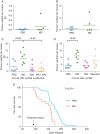
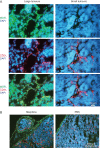
Results: Morphine did not influence tumour development when given before the onset of tumour appearance, but significantly promoted progression of established tumours, and reduced survival. MOR-immunoreactivity (ir) was observed in larger but not in smaller tumours. Morphine treatment resulted in increased tumour angiogenesis, peri-tumoural lymphangiogenesis, mast cell activation, and higher levels of cytokines and SP in tumours. SP-ir co-localized with mast cells and elsewhere in the tumours.
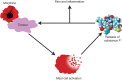
Conclusions: Morphine does not affect the onset of tumour development, but it promotes growth of existing tumours, and reduces overall survival in mice. MOR may be associated with morphine-induced cancer progression, resulting in shorter survival. Mast cell activation by morphine may contribute to increased cytokine and SP levels, leading to cancer progression and refractory pain.
Keywords: analgesics, opioid, morphine; angiogenesis, lymphangiogenesis; cancer; mast cells; μ-opioid receptor.
© The Author [2014]. Published by Oxford University Press on behalf of the British Journal of Anaesthesia. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Brogan SE, Winter NB. Patient-controlled intrathecal analgesia for the management of breakthrough cancer pain: a retrospective review and commentary. Pain Med. 2011;12:1758–68. - PubMed
-
- Greco MT, Corli O, Montanari M, et al. Epidemiology and pattern of care of breakthrough cancer pain in a longitudinal sample of cancer patients: results from the Cancer Pain Outcome Research Study Group. Clin J Pain. 2011;27:9–18. - PubMed
-
- Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–8. - PubMed
-
- Farooqui M, Geng ZH, Stephenson EJ, et al. Naloxone acts as an antagonist of estrogen receptor activity in MCF-7 cells. Mol Cancer Ther. 2006;5:611–20. - PubMed
-
- Singleton PA, Lingen MW, Fekete MJ, Garcia JG, Moss J. Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvasc Res. 2006;72:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

