Vaccines against human diarrheal pathogens: current status and perspectives
- PMID: 24861668
- PMCID: PMC5396248
- DOI: 10.4161/hv.29241
Vaccines against human diarrheal pathogens: current status and perspectives
Abstract
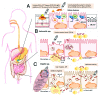
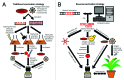
Worldwide, nearly 1.7 billion people per year contract diarrheal infectious diseases (DID) and almost 760 000 of infections are fatal. DID are a major problem in developing countries where poor sanitation prevails and food and water may become contaminated by fecal shedding. Diarrhea is caused by pathogens such as bacteria, protozoans and viruses. Important diarrheal pathogens are Vibrio cholerae, Shigella spp. and rotavirus, which can be prevented with vaccines for several years. The focus of this review is on currently available vaccines against these three pathogens, and on development of new vaccines. Currently, various types of vaccines based on traditional (killed, live attenuated, toxoid or conjugate vaccines) and reverse vaccinology (DNA/mRNA, vector, recombinant subunit, plant vaccines) are in development or already available. Development of new vaccines demands high levels of knowledge, experience, budget, and time, yet promising new vaccines often fail in preclinical and clinical studies. Efficacy of vaccination also depends on the route of delivery, and mucosal immunization in particular is of special interest for preventing DID. Furthermore, adjuvants, delivery systems and other vaccine components are essential for an adequate immune response. These aspects will be discussed in relation to the improvement of existing and development of new vaccines against DID.
Keywords: Shigellaspp. Campylobacterspp.; Vibrio cholerae; diarrheal diseases; human pathogen; oral vaccine; recombinant vaccine; rotavirus.
Figures

Similar articles
-
A review of the current status of enteric vaccines.P N G Med J. 1995 Dec;38(4):325-31. P N G Med J. 1995. PMID: 9522876 Review.
-
[Examples for vaccines against diarrheal diseases--rotavirus and traveller's diarrhea].Wien Med Wochenschr. 2007;157(5-6):102-6. doi: 10.1007/s10354-007-0390-1. Wien Med Wochenschr. 2007. PMID: 17427005 Review. German.
-
Implications and measurement of herd protection (indirect effects) for enteric vaccine development.Vaccine. 2019 Aug 7;37(34):4775-4777. doi: 10.1016/j.vaccine.2019.02.061. Vaccine. 2019. PMID: 31358237 Review.
-
[Vaccination against diarrheal diseases and typhoid fever. Current status and prospects].Ann Med Interne (Paris). 1998 Oct;149(6):340-50. Ann Med Interne (Paris). 1998. PMID: 9853044 Review. French.
-
Rotavirus vaccines: current status and future considerations.Hum Vaccin Immunother. 2014;10(6):1436-48. doi: 10.4161/hv.28857. Epub 2014 Apr 22. Hum Vaccin Immunother. 2014. PMID: 24755452 Free PMC article. Review.
Cited by
-
Recent advances in the production of recombinant subunit vaccines in Pichia pastoris.Bioengineered. 2016 Apr;7(3):155-65. doi: 10.1080/21655979.2016.1191707. Bioengineered. 2016. PMID: 27246656 Free PMC article. Review.
-
Plant-made oral vaccines against human infectious diseases-Are we there yet?Plant Biotechnol J. 2015 Oct;13(8):1056-70. doi: 10.1111/pbi.12471. Epub 2015 Sep 7. Plant Biotechnol J. 2015. PMID: 26387509 Free PMC article. Review.
-
Combination vaccines against diarrheal diseases.Hum Vaccin Immunother. 2015;11(6):1434-48. doi: 10.4161/21645515.2014.986984. Hum Vaccin Immunother. 2015. PMID: 25891647 Free PMC article. Review.
-
Association between socioeconomic deprivation and incidence of infectious intestinal disease by pathogen and linked transmission route: An ecological analysis in the UK.Epidemiol Infect. 2023 Jun 14;151:e109. doi: 10.1017/S0950268823000869. Epidemiol Infect. 2023. PMID: 37313601 Free PMC article.
-
Protective multi-epitope candidate vaccine for urinary tract infection.Biotechnol Rep (Amst). 2020 Nov 26;28:e00564. doi: 10.1016/j.btre.2020.e00564. eCollection 2020 Dec. Biotechnol Rep (Amst). 2020. PMID: 33304840 Free PMC article.
References
-
- WHO. World Health Report 2006: working together for health. Geneva: World Health Organization, 2006. - PubMed
-
- WHO. WHO fact sheet N°330. Diarrhoeal disease. WHO, 2013.
-
- Diarrhoea WHO. why children are still dying and what can be done. Geneva: World Health Organization, 2009.
-
- RehydrationProject. Why is diarrhoea dangerous?, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
