Tenofovir diphosphate concentrations and prophylactic effect in a macaque model of rectal simian HIV transmission
- PMID: 24862094
- PMCID: PMC4130383
- DOI: 10.1093/jac/dku162
Tenofovir diphosphate concentrations and prophylactic effect in a macaque model of rectal simian HIV transmission
Abstract
Objectives: This study evaluated the relationship between intracellular tenofovir diphosphate concentrations in peripheral blood mononuclear cells and prophylactic efficacy in a macaque model for HIV pre-exposure prophylaxis (PrEP).
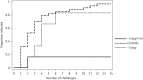
Methods: Macaques were challenged with simian HIV (SHIV) via rectal inoculation once weekly for up to 14 weeks. A control group (n=34) received no drug, a second group (n=6) received oral tenofovir disoproxil fumarate/emtricitabine 3 days before each virus challenge and a third group (n=6) received the same dosing plus another dose 2 h after virus challenge. PBMCs were collected just before each weekly virus challenge. The relationship between tenofovir diphosphate in PBMCs and prophylactic efficacy was assessed with a Cox proportional hazards model.
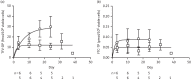
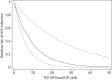
Results: The percentages of animals infected in the control, one-dose and two-dose groups were 97, 83 and 17, respectively. The mean (SD) steady-state tenofovir diphosphate concentration (fmol/10(6) cells) was 15.8 (7.6) in the one-dose group and 30.7 (10.1) in the two-dose group. Each 5 fmol tenofovir diphosphate/10(6) cells was associated with a 40% (95% CI 17%-56%) reduction in risk of SHIV acquisition, P=0.002. The tenofovir diphosphate concentration associated with a 90% reduction in risk (EC90) was 22.6 fmol/10(6) cells (95% CI 13.8-60.8).
Conclusions: The prophylactic EC90 for tenofovir diphosphate identified in macaques exposed rectally compares well with the EC90 previously identified in men who have sex with men (MSM; 16 fmol/10(6) cells, 95% CI 3-28). These results highlight the relevance of this model to inform human PrEP studies of oral tenofovir disoproxil fumarate/emtricitabine for MSM.
Keywords: HIV pre-exposure prophylaxis; intracellular pharmacology; non-human primate model; nucleoside analogues; pharmacodynamics.
© The Author 2014. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Romano J, Kashuba A, Becker S, et al. Pharmacokinetics and pharmacodynamics in HIV prevention; current status and future directions: a summary of the DAIDS and BMGF sponsored think tank on pharmacokinetics (PK)/pharmacodynamics (PD) in HIV prevention. AIDS Res Hum Retroviruses. 2013;29:1418–27. - PMC - PubMed
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

