Macrophage derived cystatin B/cathepsin B in HIV replication and neuropathogenesis
- PMID: 24862331
- PMCID: PMC4122617
- DOI: 10.2174/1570162x12666140526120249
Macrophage derived cystatin B/cathepsin B in HIV replication and neuropathogenesis
Abstract
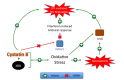
Mononuclear phagocytes including monocytes and macrophages, are important defense components of innate immunity, but can be detrimental in HIV-1 infection by serving as the principal reservoirs of virus in brain and triggering a strong immune response. These viral reservoirs represent a challenge to HIV-1 eradication since they continue producing virus in tissue despite antiretroviral therapy. HIV-1 associated neurocognitive disorders (HAND) involve alterations to the blood-brain barrier and migration of activated HIV-1 infected monocytes to the brain with subsequent induced immune activation response. Our group recently showed that HIV replication in monocyte-derived macrophages is associated with increased cystatin B. This cysteine protease inhibitor also inhibits the interferon-induced antiviral response by decreasing levels of tyrosine phosphorylated STAT-1. These recent discoveries reveal novel mechanisms of HIV persistence that could be targeted by new therapeutic approaches to eliminate HIV in macrophage reservoirs. However, cystatin B has been also associated with neuroprotection. Cystatin B is an inhibitor of the cysteine protease cathepsin B, a potent neurotoxin. During HIV-1 infection cystatin B and cathepsin B are upregulated in macrophages. Reduction in cystatin/cathepsin interactions in infected macrophages leads to increased cathepsin B secretion and activity which contributes to neuronal apoptosis. Increased intracellular expression of both proteins was recently found in monocytes from Hispanic women with HAND. These findings provide new evidence for the role of cathepsin /cystatin system in the neuropathogenesis induced by HIV-infected macrophages. We summarize recent research on cystatin B and one of its substrates, cathepsin B, in HIV replication in macrophages and neuropathogenesis.
Figures

Similar articles
-
Dysregulation of macrophage-secreted cathepsin B contributes to HIV-1-linked neuronal apoptosis.PLoS One. 2012;7(5):e36571. doi: 10.1371/journal.pone.0036571. Epub 2012 May 31. PLoS One. 2012. PMID: 22693552 Free PMC article.
-
Cathepsin B and cystatin B in HIV-seropositive women are associated with infection and HIV-1-associated neurocognitive disorders.AIDS. 2013 Jan 28;27(3):347-56. doi: 10.1097/QAD.0b013e32835b3e47. AIDS. 2013. PMID: 23291538 Free PMC article.
-
Inhibition of interferon response by cystatin B: implication in HIV replication of macrophage reservoirs.J Neurovirol. 2012 Feb;18(1):20-9. doi: 10.1007/s13365-011-0061-2. Epub 2011 Dec 7. J Neurovirol. 2012. PMID: 22147503 Free PMC article.
-
Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders.Curr HIV Res. 2014;12(2):85-96. doi: 10.2174/1570162x12666140526114526. Curr HIV Res. 2014. PMID: 24862333 Free PMC article. Review.
-
The impact of substance abuse on HIV-mediated neuropathogenesis in the current ART era.Brain Res. 2019 Dec 1;1724:146426. doi: 10.1016/j.brainres.2019.146426. Epub 2019 Aug 29. Brain Res. 2019. PMID: 31473221 Free PMC article. Review.
Cited by
-
Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-κB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages.Int J Mol Sci. 2024 Mar 13;25(6):3246. doi: 10.3390/ijms25063246. Int J Mol Sci. 2024. PMID: 38542221 Free PMC article.
-
HIV-1 Tat Promotes Lysosomal Exocytosis in Astrocytes and Contributes to Astrocyte-mediated Tat Neurotoxicity.J Biol Chem. 2016 Oct 21;291(43):22830-22840. doi: 10.1074/jbc.M116.731836. Epub 2016 Sep 8. J Biol Chem. 2016. PMID: 27609518 Free PMC article.
-
Endolysosome iron restricts Tat-mediated HIV-1 LTR transactivation by increasing HIV-1 Tat oligomerization and β-catenin expression.J Neurovirol. 2021 Oct;27(5):755-773. doi: 10.1007/s13365-021-01016-5. Epub 2021 Sep 22. J Neurovirol. 2021. PMID: 34550543 Free PMC article.
-
High levels of cathepsin D and cystatin B are associated with increased risk of coronary events.Open Heart. 2016 Jan 27;3(1):e000353. doi: 10.1136/openhrt-2015-000353. eCollection 2016. Open Heart. 2016. PMID: 26848396 Free PMC article.
-
Cocaine potentiates cathepsin B secretion and neuronal apoptosis from HIV-infected macrophages.J Neuroimmune Pharmacol. 2014 Dec;9(5):703-15. doi: 10.1007/s11481-014-9563-z. Epub 2014 Sep 11. J Neuroimmune Pharmacol. 2014. PMID: 25209871 Free PMC article.
References
-
- Turk V., Turk B., Guncar G., Turk D., Kos J. Lysosomal cathepsins: structure, role in antigen processing and presentation, and cancer. Adv. Enzyme Regul. 2002;42:285–303. - PubMed
-
- Turk B., Turk D., Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta. 2000;1477(1-2):98–111. - PubMed
-
- Turk D., Podobnik M., Kuhelj R., Dolinar M., Turk V. Crystal structures of human procathepsin B at 3.2 and 3.3 Angstroms resolution reveal an interaction motif between a papain-like cysteine protease and its propeptide. FEBS Lett. 1996;384(3):211–214. - PubMed
-
- Sakamoto M., Miyamoto K., Wu Z., Nakanishi H. Possible involvement of cathepsin B released by microglia in methylmercury-induced cerebellar pathological changes in the adult rat. Neurosci. Lett. 2008;442:292–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
