Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection
- PMID: 24862332
- PMCID: PMC4152918
- DOI: 10.2174/1570162x12666140526114956
Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection
Abstract
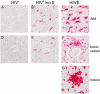
HIV-associated neurocognitive disorders (HAND) describes different levels of neurocognitive impairment, which are a common complication of HIV infection. The most severe of these, HIV-associated dementia (HIV-D), has decreased in incidence since the introduction of combination antiretroviral therapy (cART), while an increase in the less severe, minor neurocognitive disorder (MND), is now seen. The neuropathogenesis of HAND is not completely understood, however macrophages (MΦ)s/microglia are believed to play a prominent role in the development of the more severe HIV-D. Here, we report evidence of neuroinflammation in autopsy tissues from patients with HIV infection and varying degrees of neurocognitive impairment but without HIV encephalitis (HIVE). MΦ/microglial and astrocyte activation is less intense but similar to that seen in HIVE, one of the neuropathologies underlying HIV-D. MΦs and microglia appear to be activated, as determined by CD163, CD16, and HLA-DR expression, many having a rounded or ramified morphology with thickened processes, classically associated with activation. Astrocytes also show considerable morphological alterations consistent with an activated state and have increased expression of GFAP and vimentin, as compared to seronegative controls. Interestingly, in some areas, astrocyte activation appears to be limited to perivascular locations, suggesting events at the blood-brain barrier may influence astrocyte activity. In contrast to HIVE, productive HIV infection was not detectable by tyramide signal-amplified immunohistochemistry or in situ hybridization in the CNS of HIV infected persons without encephalitis. These findings suggest significant CNS inflammation, even in the absence of detectable virus production, is a common mechanism between the lesser and more severe HIV-associated neurodegenerative disease processes and supports the notion that MND and HIV-D are a continuum of the same disease.
Figures







Similar articles
-
CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE).J Neuropathol Exp Neurol. 2004 Dec;63(12):1255-64. doi: 10.1093/jnen/63.12.1255. J Neuropathol Exp Neurol. 2004. PMID: 15624762
-
Does drug abuse alter microglial phenotype and cell turnover in the context of advancing HIV infection?Neuropathol Appl Neurobiol. 2005 Jun;31(3):325-38. doi: 10.1111/j.1365-2990.2005.00648.x. Neuropathol Appl Neurobiol. 2005. PMID: 15885069
-
Impact of opiate addiction on neuroinflammation in HIV.J Neurovirol. 2012 Oct;18(5):364-73. doi: 10.1007/s13365-012-0118-x. Epub 2012 Jul 14. J Neurovirol. 2012. PMID: 22797933 Free PMC article.
-
The neuropathology of adult HIV infection.Rev Neurol (Paris). 1998 Dec;154(12):816-29. Rev Neurol (Paris). 1998. PMID: 9932303 Review.
-
When do models of NeuroAIDS faithfully imitate "the real thing"?J Neurovirol. 2018 Apr;24(2):146-155. doi: 10.1007/s13365-017-0601-5. Epub 2017 Dec 18. J Neurovirol. 2018. PMID: 29256039 Free PMC article. Review.
Cited by
-
Multimodal Investigation of Neuroinflammation in Aviremic Patients With HIV on Antiretroviral Therapy and HIV Elite Controllers.Neurol Neuroimmunol Neuroinflamm. 2022 Feb 9;9(2):e1144. doi: 10.1212/NXI.0000000000001144. Print 2022 Mar. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 35140142 Free PMC article.
-
White Matter Abnormalities Linked to Interferon, Stress Response, and Energy Metabolism Gene Expression Changes in Older HIV-Positive Patients on Antiretroviral Therapy.Mol Neurobiol. 2020 Feb;57(2):1115-1130. doi: 10.1007/s12035-019-01795-3. Epub 2019 Nov 5. Mol Neurobiol. 2020. PMID: 31691183 Free PMC article.
-
Alterations in brain TREM2 and Amyloid-β levels are associated with neurocognitive impairment in HIV-infected persons on antiretroviral therapy.J Neurochem. 2018 Dec;147(6):784-802. doi: 10.1111/jnc.14582. Epub 2018 Nov 26. J Neurochem. 2018. PMID: 30152135 Free PMC article.
-
HIV Neuropathogenesis in the Presence of a Disrupted Dopamine System.J Neuroimmune Pharmacol. 2020 Dec;15(4):729-742. doi: 10.1007/s11481-020-09927-6. Epub 2020 Jun 6. J Neuroimmune Pharmacol. 2020. PMID: 32506353 Free PMC article. Review.
-
Cortical neurons are a prominent source of the proinflammatory cytokine osteopontin in HIV-associated neurocognitive disorders.J Neurovirol. 2015 Apr;21(2):174-85. doi: 10.1007/s13365-015-0317-3. Epub 2015 Jan 31. J Neurovirol. 2015. PMID: 25636782 Free PMC article.
References
-
- Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–50. - PubMed
-
- Anthony IC, Ramage SN, Carnie FW, et al. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64(6):529–36. - PubMed
-
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7(27):1–26. - PubMed
-
- Ciardi A, Sinclair E, Scaravilli F, et al. The involvement of the cerebral cortex in human immunodeficiency virus encephalopathy: a morphological and immunohistochemical study. Acta Neuropathol. 1990;81(1):51–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
