Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice
- PMID: 24862368
- PMCID: PMC4233002
- DOI: 10.1007/s00213-014-3629-8
Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice
Abstract
Rationale: Previous studies indicate that psychosocial stressors could accelerate amyloid-β (Aβ) levels and accelerate plaque deposition in mouse models of Alzheimer disease (AD). Stressors enhanced the release of corticotrophin-releasing factor (CRF), and exogenous CRF administration mimicked the effects of stress on Aβ levels in mouse models of AD. However, whether CRF receptor 1 (CRF1) antagonists could influence the stress-induced acceleration of an AD-like process in mouse models has not been well studied.
Objective: We sought to examine whether CRF1 antagonists inhibit the effects of isolation stress on tissue Aβ levels, Aβ plaque deposition, and behaviors related to anxiety and memory in Tg2576 mice, and to investigate the molecular mechanism underlying such effects.
Methods: Cohorts of Tg2576 mouse pups were isolated or group-housed at 21 days of age, and then the subgroups of these cohorts received daily intraperitoneal injections of the CRF1 antagonists, antalarmin or R121919 (5, 10, and 20 mg/kg), or vehicle for 1 week. Other cohorts of Tg2576 mouse pups were isolated or group-housed at 21 days of age, and then at 4 months of age, subgroups of these mice were administered antalarmin (20 mg/kg) or vehicle in their drinking water for 6 months. Finally, cultured primary hippocampal neurons from regular Tg2576 pups (P0) were incubated with CRF (0.1, 1, and 10 nM), antalarmin (100 nM) or H-89 (1 μM) for 48 h. Brain tissues or cultured neurons were collected for histological and biochemical analyses, and behavioral measures were collected in the cohorts of mice that were chronically stressed.
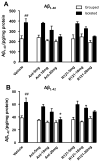
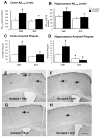
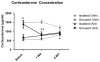
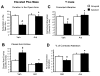
Results: Administration of antalarmin at 20 mg/kg dose for 1 week significantly reduced Aβ1-42 levels in isolation stressed mice. Administration of antalarmin for 6 months significantly decreased plasma corticosterone levels, tissue Aβ1-42 levels, and Aβ plaque deposition in the brain and blocked the effects of isolation stress on behaviors related to anxiety and memory. Finally, incubation of neurons with 100 nM antalarmin inhibited the ability of 10 nM CRF to increase Aβ1-42 levels and protein kinase A IIβ expression. The effect of CRF1 on Aβ1-42 levels was also diminished by treatment with H-89, a c-AMP/PKA inhibitor.
Conclusions: These results suggest that CRF1 antagonists can slow an AD-like process in Tg2576 mice and that the c-AMP/PKA signaling pathway may be involved in this effect.
Conflict of interest statement
The rest of the authors declare that they have no competing financial interests.
Figures
Similar articles
-
Corticotrophin releasing factor receptor 1 antagonists prevent chronic stress-induced behavioral changes and synapse loss in aged rats.Psychoneuroendocrinology. 2018 Apr;90:92-101. doi: 10.1016/j.psyneuen.2018.02.013. Epub 2018 Feb 19. Psychoneuroendocrinology. 2018. PMID: 29477954 Free PMC article.
-
Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer's disease.J Alzheimers Dis. 2012;28(3):579-92. doi: 10.3233/JAD-2011-111328. J Alzheimers Dis. 2012. PMID: 22045495 Free PMC article.
-
Corticosterone and related receptor expression are associated with increased beta-amyloid plaques in isolated Tg2576 mice.Neuroscience. 2008 Jul 31;155(1):154-63. doi: 10.1016/j.neuroscience.2008.05.017. Epub 2008 May 21. Neuroscience. 2008. PMID: 18571864 Free PMC article.
-
Don't stress about CRF: assessing the translational failures of CRF1antagonists.Psychopharmacology (Berl). 2017 May;234(9-10):1467-1481. doi: 10.1007/s00213-017-4556-2. Epub 2017 Mar 7. Psychopharmacology (Berl). 2017. PMID: 28265716 Free PMC article. Review.
-
Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications.Curr Pharm Des. 1999 May;5(5):289-315. Curr Pharm Des. 1999. PMID: 10213797 Review.
Cited by
-
Is AD a Stress-Related Disorder? Focus on the HPA Axis and Its Promising Therapeutic Targets.Front Aging Neurosci. 2019 Sep 27;11:269. doi: 10.3389/fnagi.2019.00269. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31611783 Free PMC article.
-
Corticotrophin releasing factor receptor 1 antagonists prevent chronic stress-induced behavioral changes and synapse loss in aged rats.Psychoneuroendocrinology. 2018 Apr;90:92-101. doi: 10.1016/j.psyneuen.2018.02.013. Epub 2018 Feb 19. Psychoneuroendocrinology. 2018. PMID: 29477954 Free PMC article.
-
The CRF System as a Therapeutic Target for Neuropsychiatric Disorders.Trends Pharmacol Sci. 2016 Dec;37(12):1045-1054. doi: 10.1016/j.tips.2016.09.004. Epub 2016 Oct 4. Trends Pharmacol Sci. 2016. PMID: 27717506 Free PMC article. Review.
-
The stress response neuropeptide CRF increases amyloid-β production by regulating γ-secretase activity.EMBO J. 2015 Jun 12;34(12):1674-86. doi: 10.15252/embj.201488795. Epub 2015 May 11. EMBO J. 2015. PMID: 25964433 Free PMC article.
-
Functional Impact of Corticotropin-Releasing Factor Exposure on Tau Phosphorylation and Axon Transport.PLoS One. 2016 Jan 20;11(1):e0147250. doi: 10.1371/journal.pone.0147250. eCollection 2016. PLoS One. 2016. PMID: 26790099 Free PMC article.
References
-
- Alkadhi KA. Chronic psychosocial stress exposes Alzheimer’s disease phenotype in a novel at-risk model. Front Biosci (Elite Ed) 2012;4:214–229. - PubMed
-
- Behan DP, Heinrichs SC, Troncoso JC, Liu XJ, Kawas CH, Ling N, De Souza EB. Displacement of corticotropin releasing factor from its binding protein as a possible treatment for Alzheimer’s disease. Nature. 1995;378:284–287. - PubMed
-
- Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, Stiger T. A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. Am J Psychiatry. 2008;165:617–620. - PubMed
-
- Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ, Gobeske KT, Sweatt JD, Manji HK, Arnsten AF. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. - PubMed
-
- Carroll JC, Iba M, Bangasser DA, Valentino RJ, James MJ, Brunden KR, Lee VM, Trojanowski JQ. Chronic Stress Exacerbates Tau Pathology, Neurodegeneration, and Cognitive Performance through a Corticotropin-Releasing Factor Receptor-Dependent Mechanism in a Transgenic Mouse Model of Tauopathy. J Neurosci. 2011;31:14436–14449. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

