Prostate Cancer Prevention Trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer
- PMID: 24862395
- PMCID: PMC4035700
- DOI: 10.1016/j.urology.2014.02.035
Prostate Cancer Prevention Trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer
Abstract
Objective: To modify the Prostate Cancer Prevention Trial risk calculator (PCPTRC) to predict low- vs high-grade (Gleason grade≥7) prostate cancer and incorporate percent free-prostate-specific antigen (PSA).
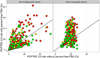
Methods: Data from 6664 Prostate Cancer Prevention Trial placebo arm biopsies (5826 individuals), where prostate-specific antigen and digital rectal examination results were available within 1 year before the biopsy and PSA was ≤10 ng/mL, were used to develop a nominal logistic regression model to predict the risk of no vs low-grade (Gleason grade<7) vs high-grade cancer (Gleason grade≥7). Percent free-PSA was incorporated into the model based on likelihood ratio analysis of a San Antonio Biomarkers of Risk cohort. Models were externally validated on 10 Prostate Biopsy Collaborative Group cohorts and 1 Early Detection Research Network reference set.
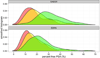
Results: Of all the Prostate Cancer Prevention Trial biopsies, 5468 (82.1%) were negative for prostate cancer, 942 (14.1%) detected low-grade, and 254 (3.8%) detected high-grade disease. Significant predictors were (log base 2) PSA (odds ratio for low-grade vs no cancer, 1.29*; high-grade vs no cancer, 2.02*; high-grade vs low-grade cancer, 1.57*), digital rectal examination (0.96, 1.49*, 1.55*, respectively), age (1.02*, 1.05*, 1.03*, respectively), African American race (1.13, 2.83*, 2.51*, respectively), prior biopsy (0.63*, 0.81, 1.27, respectively), and family history (1.31*, 1.25, 0.95, respectively), where * indicates P value<.05. The new PCPTRC 2.0 either with or without percent free-PSA (also significant by the likelihood ratio method) validated well externally.
Conclusion: By differentiating the risk of low- vs high-grade disease on biopsy, PCPTRC 2.0 better enables physician-patient counseling concerning whether to proceed to biopsy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Comment in
-
Editorial comment.Urology. 2014 Jun;83(6):1367-8. doi: 10.1016/j.urology.2014.02.036. Urology. 2014. PMID: 24862396 No abstract available.
-
Reply: To PMID 24862395.Urology. 2014 Jun;83(6):1368. doi: 10.1016/j.urology.2014.02.037. Urology. 2014. PMID: 24862397 No abstract available.
References
-
- Aizer AA, Paly JJ, Zietman AL, et al. Models of care and NCCN guideline adherence in very-low-risk prostate cancer. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. In: Thompson IM, Ankerst DP, Chi C, et al., editors. J Natl Compr Canc Netw. Vol. 11. 2013. pp. 1364–1372. - PubMed
-
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. - PubMed
-
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98:529–534. - PubMed
-
- Ankerst DP, Groskopf J, Day JR, et al. Predicting prostate cancer risk through incorporation of prostate cancer gene 3. J Urol. 2008;180:1303–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA037429/CA/NCI NIH HHS/United States
- P50 CA092629/CA/NCI NIH HHS/United States
- CA37429/CA/NCI NIH HHS/United States
- U24 CA115102/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U01CA86402/CA/NCI NIH HHS/United States
- U24CA115102/CA/NCI NIH HHS/United States
- U01 CA113913/CA/NCI NIH HHS/United States
- P30-CA008748/CA/NCI NIH HHS/United States
- P30 CA054174/CA/NCI NIH HHS/United States
- 5P30 CA054174-19/CA/NCI NIH HHS/United States
- UM1 CA182883/CA/NCI NIH HHS/United States
- P50-CA92629/CA/NCI NIH HHS/United States
- UG1 CA189974/CA/NCI NIH HHS/United States
- UM1 CA182883-01/CA/NCI NIH HHS/United States
- U01 CA086402/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

