CENP-I and Aurora B act as a molecular switch that ties RZZ/Mad1 recruitment to kinetochore attachment status
- PMID: 24862574
- PMCID: PMC4033774
- DOI: 10.1083/jcb.201307137
CENP-I and Aurora B act as a molecular switch that ties RZZ/Mad1 recruitment to kinetochore attachment status
Abstract
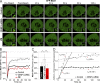
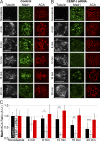
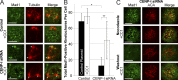
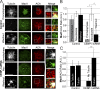
The RZZ (Rod, ZW10, and Zwilch) complex and Mad1 proteins tightly associate with kinetochores to generate the spindle checkpoint signal, but they are released when a kinetochore forms mature microtubule attachments. Here we demonstrate that the centromere protein CENP-I is required to generate a stable association of RZZ and Mad1 with kinetochores. CENP-I also inhibits their removal by dynein stripping. This regulation of Mad1 and RZZ dissociation functions independently of Aurora B, which regulates their association. We show that the microtubule status of each kinetochore independently dictates the recruitment of Aurora B kinase, kinase activity on a kinetochore substrate, and loading of spindle checkpoint proteins. This dynamic regulation of Mad1 association by Aurora B is only uncovered when CENP-I is depleted, consistent with our finding that CENP-I inhibits the dissociation of Mad1. We conclude that the dual activities of Aurora B and CENP-I generate a molecular switch that maintains a robust spindle checkpoint signal at prometaphase kinetochores until they attain mature attachments to microtubules.
© 2014 Matson and Stukenberg.
Figures



Similar articles
-
NudE regulates dynein at kinetochores but is dispensable for other dynein functions in the C. elegans early embryo.J Cell Sci. 2018 Jan 8;131(1):jcs212159. doi: 10.1242/jcs.212159. J Cell Sci. 2018. PMID: 29192061 Free PMC article.
-
A conserved CENP-E region mediates BubR1-independent recruitment to the outer corona at mitotic onset.Curr Biol. 2024 Mar 11;34(5):1133-1141.e4. doi: 10.1016/j.cub.2024.01.042. Epub 2024 Feb 13. Curr Biol. 2024. PMID: 38354735 Free PMC article.
-
Zwint-1 is a novel Aurora B substrate required for the assembly of a dynein-binding platform on kinetochores.Mol Biol Cell. 2011 Sep;22(18):3318-30. doi: 10.1091/mbc.E11-03-0213. Epub 2011 Jul 20. Mol Biol Cell. 2011. PMID: 21775627 Free PMC article.
-
Mechanisms of kinesin-7 CENP-E in kinetochore-microtubule capture and chromosome alignment during cell division.Biol Cell. 2019 Jun;111(6):143-160. doi: 10.1111/boc.201800082. Epub 2019 Feb 26. Biol Cell. 2019. PMID: 30784092 Review.
-
Spindly switch controls anaphase: spindly and RZZ functions in chromosome attachment and mitotic checkpoint control.Cell Cycle. 2011 Feb 1;10(3):449-56. doi: 10.4161/cc.10.3.14759. Epub 2011 Feb 1. Cell Cycle. 2011. PMID: 21252629 Review.
Cited by
-
Spindle Architectural Features Must Be Considered Along With Cell Size to Explain the Timing of Mitotic Checkpoint Silencing.Front Physiol. 2021 Jan 28;11:596263. doi: 10.3389/fphys.2020.596263. eCollection 2020. Front Physiol. 2021. PMID: 33584330 Free PMC article.
-
Increased Aurora B expression reduces substrate phosphorylation and induces chromosomal instability.Front Cell Dev Biol. 2022 Oct 13;10:1018161. doi: 10.3389/fcell.2022.1018161. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36313574 Free PMC article.
-
The ESCRT protein Chmp4c regulates mitotic spindle checkpoint signaling.J Cell Biol. 2018 Mar 5;217(3):861-876. doi: 10.1083/jcb.201709005. Epub 2018 Jan 23. J Cell Biol. 2018. PMID: 29362225 Free PMC article.
-
Structure of the RZZ complex and molecular basis of its interaction with Spindly.J Cell Biol. 2017 Apr 3;216(4):961-981. doi: 10.1083/jcb.201611060. Epub 2017 Mar 20. J Cell Biol. 2017. PMID: 28320825 Free PMC article.
-
RZZ-SPINDLY-DYNEIN: you got to keep 'em separated.Cell Cycle. 2020 Jul;19(14):1716-1726. doi: 10.1080/15384101.2020.1780382. Epub 2020 Jun 16. Cell Cycle. 2020. PMID: 32544383 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

