Cell proliferation in in vivo-like three-dimensional cell culture is regulated by sequestration of ERK1/2 to lipid rafts
- PMID: 24862604
- PMCID: PMC6496208
- DOI: 10.1111/cpr.12112
Cell proliferation in in vivo-like three-dimensional cell culture is regulated by sequestration of ERK1/2 to lipid rafts
Abstract
Objectives: Regulatory mechanisms of cell proliferation have been extensively studied as they represent major challenges when dealing with pathologies such as fibrosis, tumourigenesis or tissue regeneration. Numerous in vitro studies still exploit conventional, two-dimensional cell cultures where cells are forced to adhere to unnaturally stiff and flat surfaces of culture dishes. In the living organism, however, each cell is in contact with components of the extracellular matrix and/or neighbouring cells, thus creating a complex three-dimensional (3D) tissue structure. The current paper describes a native 3D culture of cells, based on the GD25β1 fibroblast cell line, and its use for investigating cell proliferation in in vivo-like conditions.
Materials and methods: Four-day post-confluent culture of GD25β1 fibroblasts resulted in formation of a 3D system of cells embedded in naturally synthesized extracellular matrix. Morphological characterization of the culture was performed by histochemistry, immunohistochemistry and immunofluorescence. Viability/proliferation was assayed by MTT testing, FACS analysis and Western blotting for determination of expression levels and activation status of the relevant signalling molecules.
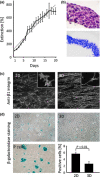
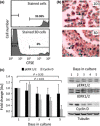
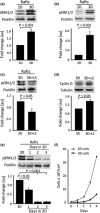
Results: GD25b1 fibroblasts, grown as 3D culture, gave rise to tissue-like structures characterized by low level of apoptosis, low senescence and development of 3D matrix adhesions, typical of living tissues. Transition to three-dimensionality led to a switch from exponential to linear culture growth, accompanied by accumulation of activated ERK1/2 into caveolin-containing raft domains. Disruption of raft domains as well as reverse transition from 3D back to monolayer culture led to release of phosphorylated ERK1/2 from rafts, activation of cyclin D1 expression and increase in proliferation levels.
Conclusions: These results imply that under in vivo-like conditions, cells might achieve reduction of their proliferation level by sequestering activated ERK1/2 to lipid rafts.
© 2014 John Wiley & Sons Ltd.
Figures
Similar articles
-
Three-dimensional matrix induces sustained activation of ERK1/2 via Src/Ras/Raf signaling pathway.Cell Biol Int. 2008 Feb;32(2):229-34. doi: 10.1016/j.cellbi.2007.08.029. Epub 2007 Sep 7. Cell Biol Int. 2008. PMID: 17933561
-
Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963
-
The hyaluronan-binding protease upregulates ERK1/2 and PI3K/Akt signalling pathways in fibroblasts and stimulates cell proliferation and migration.Cell Signal. 2005 Dec;17(12):1486-94. doi: 10.1016/j.cellsig.2005.03.007. Epub 2005 Apr 19. Cell Signal. 2005. PMID: 16153533
-
Growth and survival signalling in B16F10 melanoma cells in 3D culture.Cell Biol Int. 2010 Mar 8;34(4):385-91. doi: 10.1042/CBI20090147. Cell Biol Int. 2010. PMID: 20015052
-
Advancements in Three Dimensional In-Vitro Cell Culture Models.Chem Rec. 2022 Sep;22(9):e202200058. doi: 10.1002/tcr.202200058. Epub 2022 Jun 14. Chem Rec. 2022. PMID: 35701102 Review.
Cited by
-
Alginate-Based 3D A549 Cell Culture Model to Study Paracoccidioides Infection.J Fungi (Basel). 2023 May 31;9(6):634. doi: 10.3390/jof9060634. J Fungi (Basel). 2023. PMID: 37367570 Free PMC article.
-
The characteristics of Ishikawa endometrial cancer cells are modified by substrate topography with cell-like features and the polymer surface.Int J Nanomedicine. 2015 Aug 3;10:4883-95. doi: 10.2147/IJN.S86336. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26346435 Free PMC article.
References
-
- Evangelatov A, Pankov R (2013) The evolution of three‐dimensional cell cultures towards unimpeded regenerative medicine and tissue engineering. In: Andrades JA, ed. Regenerative Medicine and Tissue Engineering, ISBN: 978‐953‐51‐1108‐5, InTech. http://www.intechopen.com/books/regenerative-medicine-and-tissueengineer... (accessed 8 May 2014).
-
- Cukierman E, Pankov R, Stevens DR, Yamada KM (2001) Taking cell‐matrix adhesions to the third dimension. Science 294, 1708–1712. - PubMed
-
- Ahlfors J‐EW, Billiar KL (2007) Biomechanical and biochemical characteristics of a human fibroblast‐produced and remodeled matrix. Biomaterials 28, 2183–2191. - PubMed
-
- Clark RA, McCoy GA, Folkvord JM, McPherson JM (1997) TGF‐beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue‐like fibroplasia: a fibronectin matrix‐dependent event. J. Cell. Physiol. 170, 69–80. - PubMed
-
- Cukierman E, Pankov R, Yamada KM (2002) Cell interactions with three‐dimensional matrices. Curr. Opin. Cell Biol. 14, 633–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

