Age-dependent and differential effects of Smad7ΔEx1 on neural progenitor cell proliferation and on neurogenesis
- PMID: 24862634
- PMCID: PMC4162458
- DOI: 10.1016/j.exger.2014.05.011
Age-dependent and differential effects of Smad7ΔEx1 on neural progenitor cell proliferation and on neurogenesis
Abstract
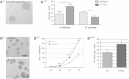
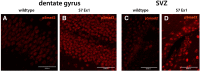
We recently reported that young (3 to 4months old) mice lacking Exon 1 of the Smad7 gene (S7ΔEx1 mice) show enhanced proliferation of neural stem and progenitor cells (NPCs) in the hippocampal dentate gyrus (DG) and in the subventricular zone (SVZ) of the lateral ventricles. It remained unclear, however, whether this phenotype would persist along aging, the latter typically being associated with a profound decrease in neurogenesis. Analysis of NPCs' proliferation based on the cell cycle marker PCNA in 12month-old S7ΔEx1 mice revealed a reversal of the phenotype. Hence, in contrast to their younger counterparts, 12month-old S7ΔEx1 mice had a reduced number of proliferating cells, compared to wildtype (WT) mice. At the same time, the survival of newly generated cells was enhanced in the aged transgenic animals. 12month-old S7ΔEx1 mice further displayed a reduced level of neurogenesis based on the numbers of cells expressing doublecortin (DCX), a marker for newborn neurons. The reduced neurogenesis in aged S7ΔEx1 mice was not due to a stem cell depletion, which might have occurred as a consequence of hyperproliferation in the young mice, since the number of Nestin and Sox2 positive cells was similar in WT and S7ΔEx1 mice. Instead, Nestin positive cells in the DG as well as primary neurosphere cultures derived from 12month-old S7ΔEx1 mice had a reduced capability to proliferate. However, after passaging, when released from their age- and niche-associated proliferative block, neurospheres from aged S7ΔEx1 mice regained the hyperproliferative property. Further, pSmad2 antibody staining intensity was elevated in the DG and SVZ of 12-month old transgenic compared to WT mice, indicating increased intracellular TGF-beta signaling in the aged S7ΔEx1 mice. In summary, this points toward differential effects of S7ΔEx1 on neurogenesis: (i) a hyperproliferation in young animals caused by a cell autonomous mechanism, and (ii) a TGF-beta dependent modulation of neurogenesis in aged S7ΔEx1 animals that abrogates the cell-intrinsic hyperproliferative properties and results in reduced proliferation, increased stem cell quiescence, and enhanced survival of newly generated cells.
Keywords: Aging; Neural stem cell; Neurogenesis; Proliferation; Smad; TGF-beta signaling.
Copyright © 2014. Published by Elsevier Inc.
Figures

Similar articles
-
Physical exercise rescues defective neural stem cells and neurogenesis in the adult subventricular zone of Btg1 knockout mice.Brain Struct Funct. 2017 Aug;222(6):2855-2876. doi: 10.1007/s00429-017-1376-4. Epub 2017 Feb 28. Brain Struct Funct. 2017. PMID: 28247022
-
Ras-GRF2 regulates nestin-positive stem cell density and onset of differentiation during adult neurogenesis in the mouse dentate gyrus.Mol Cell Neurosci. 2017 Dec;85:127-147. doi: 10.1016/j.mcn.2017.09.006. Epub 2017 Sep 28. Mol Cell Neurosci. 2017. PMID: 28966131
-
TGF-β superfamily gene expression and induction of the Runx1 transcription factor in adult neurogenic regions after brain injury.PLoS One. 2013;8(3):e59250. doi: 10.1371/journal.pone.0059250. Epub 2013 Mar 21. PLoS One. 2013. PMID: 23555640 Free PMC article.
-
Endogenous neural precursor cells in health and disease.Brain Res. 2020 Mar 1;1730:146619. doi: 10.1016/j.brainres.2019.146619. Epub 2019 Dec 24. Brain Res. 2020. PMID: 31874148 Review.
-
Development and aging of a brain neural stem cell niche.Exp Gerontol. 2017 Aug;94:9-13. doi: 10.1016/j.exger.2016.11.007. Epub 2016 Nov 17. Exp Gerontol. 2017. PMID: 27867091 Free PMC article. Review.
Cited by
-
Investigation of the relationship between chronic montelukast treatment, asthma and depression-like behavior in mice.Exp Ther Med. 2021 Jan;21(1):27. doi: 10.3892/etm.2020.9459. Epub 2020 Nov 9. Exp Ther Med. 2021. PMID: 33262813 Free PMC article.
-
Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells.Sci Rep. 2014 Nov 21;4:7144. doi: 10.1038/srep07144. Sci Rep. 2014. PMID: 25413454 Free PMC article.
-
Efficient generation of functional Schwann cells from adipose-derived stem cells in defined conditions.Cell Cycle. 2017 May 3;16(9):841-851. doi: 10.1080/15384101.2017.1304328. Epub 2017 Mar 15. Cell Cycle. 2017. PMID: 28296571 Free PMC article.
-
Uncovering the regeneration strategies of zebrafish organs: a comprehensive systems biology study on heart, cerebellum, fin, and retina regeneration.BMC Syst Biol. 2018 Mar 19;12(Suppl 2):29. doi: 10.1186/s12918-018-0544-3. BMC Syst Biol. 2018. PMID: 29560825 Free PMC article.
-
Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-β mRNA Levels Accurately Predict Treatment Response in Depressed Patients.Int J Neuropsychopharmacol. 2016 Sep 30;19(10):pyw045. doi: 10.1093/ijnp/pyw045. Print 2016 Oct. Int J Neuropsychopharmacol. 2016. PMID: 27207917 Free PMC article. Clinical Trial.
References
-
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. - PubMed
-
- Couillard-Despres S., Winner B., Schaubeck S., Aigner R., Vroemen M., Weidner N., Bogdahn U., Winkler J., Kuhn H.G., Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. - PubMed
-
- Couillard-Despres S., Wuertinger C., Kandasamy M., Caioni M., Stadler K., Aigner R., Bogdahn U., Aigner L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry. 2009;14:856–864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

