Antitumor immunity induced after α irradiation
- PMID: 24862758
- PMCID: PMC4094834
- DOI: 10.1016/j.neo.2014.04.002
Antitumor immunity induced after α irradiation
Abstract
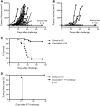
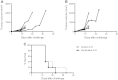
Radioimmunotherapy (RIT) is a therapeutic modality that allows delivering of ionizing radiation directly to targeted cancer cells. Conventional RIT uses β-emitting radioisotopes, but recently, a growing interest has emerged for the clinical development of α particles. α emitters are ideal for killing isolated or small clusters of tumor cells, thanks to their specific characteristics (high linear energy transfer and short path in the tissue), and their effect is less dependent on dose rate, tissue oxygenation, or cell cycle status than γ and X rays. Several studies have been performed to describe α emitter radiobiology and cell death mechanisms induced after α irradiation. But so far, no investigation has been undertaken to analyze the impact of α particles on the immune system, when several studies have shown that external irradiation, using γ and X rays, can foster an antitumor immune response. Therefore, we decided to evaluate the immunogenicity of murine adenocarcinoma MC-38 after bismuth-213 ((213)Bi) irradiation using a vaccination approach. In vivo studies performed in immunocompetent C57Bl/6 mice induced a protective antitumor response that is mediated by tumor-specific T cells. The molecular mechanisms potentially involved in the activation of adaptative immunity were also investigated by in vitro studies. We observed that (213)Bi-treated MC-38 cells release "danger signals" and activate dendritic cells. Our results demonstrate that α irradiation can stimulate adaptive immunity, elicits an efficient antitumor protection, and therefore is an immunogenic cell death inducer, which provides an attractive complement to its direct cytolytic effect on tumor cells.
Copyright © 2014 Neoplasia Press, Inc. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Goldberg Z., Lehnert B.E. Radiation-induced effects in unirradiated cells: a review and implications in cancer. Int J Oncol. 2002;21:337–349. - PubMed
-
- Morgan W.F. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene. 2003;22:7094–7099. - PubMed
-
- Mothersill C., Seymour C.B. Radiation-induced bystander effects—implications for cancer. Nat Rev Cancer. 2004;4:158–164. - PubMed
-
- Barbet J., Bardiès M., Bourgeois M., Chatal J.F., Chérel M., Davodeau F., Faivre-Chauvet A., Gestin J.F., Kraeber-Bodéré F. Radiolabeled antibodies for cancer imaging and therapy. Methods Mol Biol. 2012;907:681–697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

