Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling
- PMID: 24863258
- PMCID: PMC4099517
- DOI: 10.1016/j.bcp.2014.05.010
Cell type-specific recycling of tetrahydrobiopterin by dihydrofolate reductase explains differential effects of 7,8-dihydrobiopterin on endothelial nitric oxide synthase uncoupling
Abstract
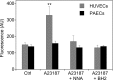
(6R)-5,6,7,8-Tetrahydro-L-biopterin (BH4) availability regulates nitric oxide and superoxide formation by endothelial nitric oxide synthase (eNOS). At low BH4 or low BH4 to 7,8-dihydrobiopterin (BH2) ratios the enzyme becomes uncoupled and generates superoxide at the expense of NO. We studied the effects of exogenously added BH2 on intracellular BH4/BH2 ratios and eNOS activity in different types of endothelial cells. Incubation of porcine aortic endothelial cells with BH2 increased BH4/BH2 ratios from 8.4 (controls) and 0.5 (BH4-depleted cells) up to ~20, demonstrating efficient reduction of BH2. Uncoupled eNOS activity observed in BH4-depleted cells was prevented by preincubation with BH2. Recycling of BH4 was much less efficient in human endothelial cells isolated from umbilical veins or derived from dermal microvessels (HMEC-1 cells), which exhibited eNOS uncoupling and low BH4/BH2 ratios under basal conditions and responded to exogenous BH2 with only moderate increases in BH4/BH2 ratios. The kinetics of dihydrofolate reductase-catalyzed BH4 recycling in endothelial cytosols showed that the apparent BH2 affinity of the enzyme was 50- to 300-fold higher in porcine than in human cell preparations. Thus, the differential regulation of eNOS uncoupling in different types of endothelial cells may be explained by striking differences in the apparent BH2 affinity of dihydrofolate reductase.
Keywords: Dihydrofolate reductase; Endothelial nitric oxide synthase uncoupling; Human endothelial cells; Porcine endothelial cells; Tetrahydrobiopterin recycling.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Gorren A.C.F., Mayer B. Nitric-oxide synthase: a cytochrome P450 family foster child. Biochim Biophys Acta. 2007;1770:432–445. - PubMed
-
- Schmidt T.S., Alp N.J. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. - PubMed
-
- Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

