Virtual Electrode Recording Tool for EXtracellular potentials (VERTEX): comparing multi-electrode recordings from simulated and biological mammalian cortical tissue
- PMID: 24863422
- PMCID: PMC4481302
- DOI: 10.1007/s00429-014-0793-x
Virtual Electrode Recording Tool for EXtracellular potentials (VERTEX): comparing multi-electrode recordings from simulated and biological mammalian cortical tissue
Abstract
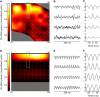
Local field potentials (LFPs) sampled with extracellular electrodes are frequently used as a measure of population neuronal activity. However, relating such measurements to underlying neuronal behaviour and connectivity is non-trivial. To help study this link, we developed the Virtual Electrode Recording Tool for EXtracellular potentials (VERTEX). We first identified a reduced neuron model that retained the spatial and frequency filtering characteristics of extracellular potentials from neocortical neurons. We then developed VERTEX as an easy-to-use Matlab tool for simulating LFPs from large populations (>100,000 neurons). A VERTEX-based simulation successfully reproduced features of the LFPs from an in vitro multi-electrode array recording of macaque neocortical tissue. Our model, with virtual electrodes placed anywhere in 3D, allows direct comparisons with the in vitro recording setup. We envisage that VERTEX will stimulate experimentalists, clinicians, and computational neuroscientists to use models to understand the mechanisms underlying measured brain dynamics in health and disease.
Figures








References
-
- Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. J Neurosci. 2007;27(35):9247–9251. doi: 10.1523/JNEUROSCI.2055-07.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

