Detergent screening of the human voltage-gated proton channel using fluorescence-detection size-exclusion chromatography
- PMID: 24863684
- PMCID: PMC4116661
- DOI: 10.1002/pro.2492
Detergent screening of the human voltage-gated proton channel using fluorescence-detection size-exclusion chromatography
Abstract
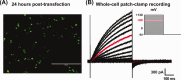
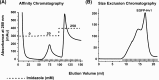
The human voltage-gated proton channel (Hv1) is a membrane protein consisting of four transmembrane domains and intracellular amino- and carboxy-termini. The protein is activated by membrane depolarization, similar to other voltage-sensitive proteins. However, the Hv1 proton channel lacks a traditional ion pore. The human Hv1 proton channel has been implicated in mediating sperm capacitance, stroke, and most recently as a biomarker/mediator of cancer metastasis. Recently, the three-dimensional structures for homologues of this voltage-gated proton channel were reported. However, it is not clear what artificial environment is needed to facilitate the isolation and purification of the human Hv1 proton channel for structural study. In the present study, we generated a chimeric protein that placed an enhanced green fluorescent protein (EGFP) to the amino-terminus of the human Hv1 proton channel (termed EGFP-Hv1). The chimeric protein was expressed in a baculovirus expression system using Sf9 cells and subjected to detergent screening using fluorescence-detection size-exclusion chromatography. The EGFP-Hv1 proton channel can be solubilized in the zwitterionic detergent Anzergent 3-12 and the nonionic n-dodecyl-β-d-maltoside (DDM) with little protein aggregation and a prominent monomeric protein peak at 48 h postinfection. Furthermore, we demonstrate that the chimeric protein exhibits a monomeric protein peak, which is distinguishable from protein aggregates, at the final size-exclusion chromatography purification step. Taken together, we can conclude that solubilization in DDM will provide a useable final product for further structural characterization of the full-length human Hv1 proton channel.
Keywords: detergent screen; electrophysiology; fluorescent-detection size-exclusion chromatography; voltage-gated proton channel.
© 2014 The Protein Society.
Figures




Similar articles
-
The role and structure of the carboxyl-terminal domain of the human voltage-gated proton channel Hv1.J Biol Chem. 2010 Apr 16;285(16):12047-54. doi: 10.1074/jbc.M109.040360. Epub 2010 Feb 10. J Biol Chem. 2010. PMID: 20147290 Free PMC article.
-
Trp207 regulation of voltage-dependent activation of human Hv1 proton channel.J Biol Chem. 2024 Mar;300(3):105674. doi: 10.1016/j.jbc.2024.105674. Epub 2024 Jan 23. J Biol Chem. 2024. PMID: 38272234 Free PMC article.
-
Proton pump inhibitors have pH-dependent effects on the thermostability of the carboxyl-terminal domain of voltage-gated proton channel Hv1.Eur Biophys J. 2018 Apr;47(3):237-247. doi: 10.1007/s00249-017-1253-3. Epub 2017 Sep 9. Eur Biophys J. 2018. PMID: 28889176
-
Voltage-gated proton (H(v)1) channels, a singular voltage sensing domain.FEBS Lett. 2015 Nov 14;589(22):3471-8. doi: 10.1016/j.febslet.2015.08.003. Epub 2015 Aug 18. FEBS Lett. 2015. PMID: 26296320 Review.
-
Gating mechanisms of voltage-gated proton channels.Annu Rev Biochem. 2015;84:685-709. doi: 10.1146/annurev-biochem-060614-034307. Annu Rev Biochem. 2015. PMID: 26034892 Review.
Cited by
-
A novel method for expressing and purifying large quantities of functional and stable human voltage-gated proton channel (hHv1).Protein Sci. 2025 Feb;34(2):e70017. doi: 10.1002/pro.70017. Protein Sci. 2025. PMID: 39865375
References
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. - PubMed
-
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. - PubMed
-
- Tate CG. Overexpression of mammalian integral membrane proteins for structural studies. FEBS Lett. 2001;504:94–98. - PubMed
-
- Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–681. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

