Efficient synthesis of 3H-indoles enabled by the lead-mediated α-arylation of β-ketoesters or γ-lactams using aryl azides
- PMID: 24865180
- PMCID: PMC4059265
- DOI: 10.1021/ol5010615
Efficient synthesis of 3H-indoles enabled by the lead-mediated α-arylation of β-ketoesters or γ-lactams using aryl azides
Abstract
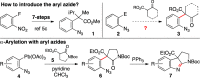
The development of a lead-mediated α-arylation reaction between aryl azides and β-ketoesters or γ-lactams that facilitates the formation of 3H-indoles is disclosed. Twenty-five examples are included which demonstrate the generality of this reaction to access aryl azides bearing tetrasubstituted o-alkyl substituents. When paired with a Staudinger reduction, this reaction streamlines the synthesis of functionalized 3H-indoles.
Figures
Similar articles
-
Rh2(II)-catalyzed ester migration to afford 3H-indoles from trisubstituted styryl azides.Org Lett. 2015 Feb 20;17(4):802-5. doi: 10.1021/ol503541z. Epub 2015 Feb 2. Org Lett. 2015. PMID: 25642741
-
Ruthenium-catalyzed γ-carbolinium ion formation from aryl azides; synthesis of dimebolin.Org Lett. 2011 May 20;13(10):2726-9. doi: 10.1021/ol2008268. Epub 2011 Apr 25. Org Lett. 2011. PMID: 21517088 Free PMC article.
-
N-heterocyclic carbene-catalyzed homoenolate additions with N-aryl ketimines as electrophiles: efficient synthesis of spirocyclic γ-lactam oxindoles.Chemistry. 2012 Jul 23;18(30):9198-203. doi: 10.1002/chem.201201375. Epub 2012 Jun 26. Chemistry. 2012. PMID: 22736551
-
Enantioselective iron-catalyzed azidation of β-keto esters and oxindoles.J Am Chem Soc. 2013 Apr 10;135(14):5356-9. doi: 10.1021/ja402082p. Epub 2013 Apr 2. J Am Chem Soc. 2013. PMID: 23537339
-
Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 2-Azides, Phthalocyanines, Subphthalocyanines and Porphyrazines.Molecules. 2020 Apr 10;25(7):1745. doi: 10.3390/molecules25071745. Molecules. 2020. PMID: 32290240 Free PMC article. Review.
Cited by
-
Synthesis and Evaluation of the Tetracyclic Ring-System of Isocryptolepine and Regioiso-Mers for Antimalarial, Antiproliferative and Antimicrobial Activities.Molecules. 2021 May 30;26(11):3268. doi: 10.3390/molecules26113268. Molecules. 2021. PMID: 34070798 Free PMC article.
References
-
-
For recent leading reviews, see:
- Gribble G. W. J. Chem. Soc., Perkin Trans. 1 2000, 1045.
- Cacchi S.; Fabrizi G. Chem. Rev. 2005, 105, 2873. - PubMed
- Knölker H.-J.; Reddy K. R.. The Alkaloids: Chemistry and Biology; Cordell G. A., Ed.; Academic Press: New York, 2008; Vol. 65, pp 1–430. - PubMed
- Edwankar C. R.; Edwankar R. V.; Namjoshi O. A.; Rallapappi S. K.; Yang S. J.; Cook J. M. Curr. Opin. Drug Discovery Dev. 2009, 12, 752. - PubMed
- Taber D. F.; Tirunahari P. K. Tetrahedron 2011, 67, 7195. - PMC - PubMed
- Schmidt A. W.; Reddy K. R.; Knölker H.-J. Chem. Rev. 2012, 112, 3193. - PubMed
-
-
-
For recent, leading papers on their applications in electronic materials, see:
- van Addy D.; Bastiaansen J. J. A. M.; Kiggen N. M. M.; Langeveld B. M. W.; Rothe C.; Monkman A.; Bach I.; Stössel P.; Brunner K. J. Am. Chem. Soc. 2004, 126, 7718. - PubMed
- Wu Y.; Li Y.; Gardner S.; Ong B. S. J. Am. Chem. Soc. 2005, 127, 614. - PubMed
- Boudreault P.-L. T.; Wakim S.; Blouin N.; Simard M.; Tessier C.; Tao Y.; Leclerc M. J. Am. Chem. Soc. 2007, 129, 9125. - PubMed
- Wang C.; Dong H.; Hu W.; Liu Y.; Zhu D. Chem. Rev. 2011, 112, 2208. - PubMed
-
-
-
cf.
- Stokes B. J.; Dong H.; Leslie B. E.; Pumphrey A. L.; Driver T. G. J. Am. Chem. Soc. 2007, 129, 7500. - PubMed
- Shen M.; Leslie B. E.; Driver T. G. Angew. Chem., Int. Ed. 2008, 47, 5056. - PubMed
- Stokes B. J.; Richert K. J.; Driver T. G. J. Org. Chem. 2009, 74, 6442. - PMC - PubMed
- Pumphrey A. L.; Dong H.; Driver T. G. Angew. Chem., Int. Ed. 2012, 51, 5920. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources