Phospholipase C-related catalytically inactive protein participates in the autophagic elimination of Staphylococcus aureus infecting mouse embryonic fibroblasts
- PMID: 24865216
- PMCID: PMC4035314
- DOI: 10.1371/journal.pone.0098285
Phospholipase C-related catalytically inactive protein participates in the autophagic elimination of Staphylococcus aureus infecting mouse embryonic fibroblasts
Abstract
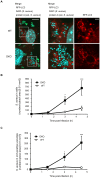
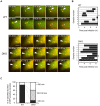
Autophagy is an intrinsic host defense system that recognizes and eliminates invading bacterial pathogens. We have identified microtubule-associated protein 1 light chain 3 (LC3), a hallmark of autophagy, as a binding partner of phospholipase C-related catalytically inactive protein (PRIP) that was originally identified as an inositol trisphosphate-binding protein. Here, we investigated the involvement of PRIP in the autophagic elimination of Staphylococcus aureus in infected mouse embryonic fibroblasts (MEFs). We observed significantly more LC3-positive autophagosome-like vacuoles enclosing an increased number of S. aureus cells in PRIP-deficient MEFs than control MEFs, 3 h and 4.5 h post infection, suggesting that S. aureus proliferates in LC3-positive autophagosome-like vacuoles in PRIP-deficient MEFs. We performed autophagic flux analysis using an mRFP-GFP-tagged LC3 plasmid and found that autophagosome maturation is significantly inhibited in PRIP-deficient MEFs. Furthermore, acidification of autophagosomes was significantly inhibited in PRIP-deficient MEFs compared to the wild-type MEFs, as determined by LysoTracker staining and time-lapse image analysis performed using mRFP-GFP-tagged LC3. Taken together, our data show that PRIP is required for the fusion of S. aureus-containing autophagosome-like vacuoles with lysosomes, indicating that PRIP is a novel modulator in the regulation of the innate immune system in non-professional phagocytic host cells.
Conflict of interest statement
Figures


Similar articles
-
Phospholipase C-related catalytically inactive protein, a novel microtubule-associated protein 1 light chain 3-binding protein, negatively regulates autophagosome formation.Biochem Biophys Res Commun. 2013 Mar 8;432(2):268-74. doi: 10.1016/j.bbrc.2013.01.119. Epub 2013 Feb 9. Biochem Biophys Res Commun. 2013. PMID: 23399561
-
The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils.Autophagy. 2021 Apr;17(4):888-902. doi: 10.1080/15548627.2020.1739443. Epub 2020 Mar 15. Autophagy. 2021. PMID: 32174246 Free PMC article.
-
Staphylococcus aureus Alpha-Toxin Induces the Formation of Dynamic Tubules Labeled with LC3 within Host Cells in a Rab7 and Rab1b-Dependent Manner.Front Cell Infect Microbiol. 2017 Oct 4;7:431. doi: 10.3389/fcimb.2017.00431. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29046869 Free PMC article.
-
Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells.J Cell Sci. 2014 Sep 15;127(Pt 18):4089-102. doi: 10.1242/jcs.156034. Epub 2014 Jul 22. J Cell Sci. 2014. PMID: 25052093
-
Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells.Autophagy. 2010 Jan;6(1):110-25. doi: 10.4161/auto.6.1.10698. Autophagy. 2010. PMID: 20110774
Cited by
-
Phospholipase C-related catalytically inactive protein can regulate obesity, a state of peripheral inflammation.Jpn Dent Sci Rev. 2017 Feb;53(1):18-24. doi: 10.1016/j.jdsr.2016.06.001. Epub 2016 Jun 27. Jpn Dent Sci Rev. 2017. PMID: 28408965 Free PMC article. Review.
-
Phospholipase C-related Catalytically Inactive Protein Is a New Modulator of Thermogenesis Promoted by β-Adrenergic Receptors in Brown Adipocytes.J Biol Chem. 2016 Feb 19;291(8):4185-96. doi: 10.1074/jbc.M115.705723. Epub 2015 Dec 25. J Biol Chem. 2016. PMID: 26706316 Free PMC article.
-
Autophagy in Staphylococcus aureus Infection.Front Cell Infect Microbiol. 2021 Oct 7;11:750222. doi: 10.3389/fcimb.2021.750222. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34692566 Free PMC article. Review.
-
Suppression of cell migration by phospholipase C-related catalytically inactive protein-dependent modulation of PI3K signalling.Sci Rep. 2017 Jul 14;7(1):5408. doi: 10.1038/s41598-017-05908-7. Sci Rep. 2017. PMID: 28710365 Free PMC article.
References
-
- Umebayashi H, Mizokami A, Matsuda M, Harada K, Takeuchi H, et al. (2013) PLC-related catalytically inactive protein (PRIP), a novel microtubule-associated protein 1 light chain 3 (LC3)-binding protein, negatively regulates autophagosome formation. Biochem Biophys Res Commun 432: 268–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

