Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews
- PMID: 24865446
- PMCID: PMC6469574
- DOI: 10.1002/14651858.CD009108.pub2
Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews
Abstract
Background: Migraine is a highly disabling condition for the individual and also has wide-reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family. It is available for administration by four different routes: oral, subcutaneous, intranasal, and rectal.
Objectives: To summarise evidence from four Cochrane intervention reviews on the efficacy and tolerability of sumatriptan in the treatment of acute migraine attacks in adults by four routes of administration (oral, subcutaneous, intranasal, and rectal) compared with both placebo and active comparators.
Methods: The included reviews were written by the authors of this overview; no additional searching was carried out. All included reviews were conducted according to a standard protocol and reported a standard set of outcomes. From each individual review we extracted results for pain relief at different levels, and adverse events. No additional statistical comparison was undertaken as part of the overview. We focused on the most important findings for doses and routes licensed in North America or Europe (oral 25 mg, 50 mg, 100 mg; subcutaneous 4 mg, 6 mg; intranasal 5 mg, 10 mg, 20 mg; rectal 25 mg).
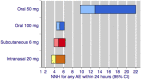
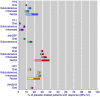
Main results: Included reviews provided data for 18 different dose and route of administration combinations in 52,236 participants. Data for the primary outcomes sought were generally well reported, and involved adequate numbers of participants to give confidence in the results, except for the rectal route of administration, where numbers were low.Subcutaneous administration was the most effective, with pain reduced from moderate or severe to none by two hours in almost 6 in 10 people (59%) taking 6 mg sumatriptan, compared with approximately 1 in 7 (15%) taking placebo; the number needed to treat (NNT) was 2.3 (95% confidence interval 2.1 to 2.4) with 2522 participants in the analysis. The most commonly used doses of oral, rectal, and intranasal sumatriptan also provided clinically useful pain relief, with the oral 50 mg dose providing complete relief of pain in almost 3 in 10 people (28%) compared with about 1 in 10 (11%) after placebo (NNT 6.1 (5.5 to 6.9) in 6447 participants). Subcutaneous administration provided more rapid pain relief than the other routes. Taking medication early, when pain was mild, was more effective than waiting until the pain was moderate or severe.The most effective dose of sumatriptan for each route of administration for the outcome of headache relief (pain reduced from moderate or severe to none or mild) at two hours was oral 100 mg (NNT 3.5 (3.2 to 3.7) in 7811 participants), subcutaneous 6 mg (NNT 2.1 (2.0 to 2.2) in 2738 participants), intranasal 20 mg (NNT 3.5 (3.1 to 4.1) in 2020 participants), and rectal 25 mg (NNT 2.4 (1.9 to 3.4) in 240 participants).Adverse events were generally of mild or moderate severity, of short duration, and more common with subcutaneously administered sumatriptan and higher doses of oral and intranasal sumatriptan than with other dose and route combinations.
Authors' conclusions: Sumatriptan is an effective abortive treatment for acute migraine attacks, but is associated with increased adverse events relative to placebo. The route of administration influences efficacy, particularly within the first hour after administration. Subcutaneous sumatriptan shows the greatest efficacy in terms of pain relief, but at the expense of relatively high levels of adverse events, and with a high financial cost compared with other routes. Information about the relative efficacy of the different routes of administration for different outcomes should help to inform decisions about the suitability of sumatriptan as a migraine treatment, as well as about the most appropriate way to administer the treatment for individual patients.
Conflict of interest statement
The authors of this overview are also the authors of the four included individual reviews.
SD and RAM have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. Support for this review came from the Oxford Pain Relief Trust.
Figures



Update of
- doi: 10.1002/14651858.CD009108
References
References to included reviews
Derry 2012a
Derry 2012b
Derry 2012c
Additional references
Ayzenberg 2012
Bendtsen 2003
-
- Bendtsen L, Mattsson P, Zwart JA, Lipton RB. Placebo response in clinical randomized trials of analgesics in migraine. Cephalalgia 2003;23(7):487‐90. - PubMed
Bigal 2003
-
- Bigal ME, Bordini CA, Antoniazzi AL, Speciali JG. The triptan formulations: a critical evaluation. Arquivos de Neuro‐Psiquiatria 2003;61(2A):313‐20. - PubMed
Bigal 2008
Bloudek 2012
-
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361‐78. [DOI: 10.1007/s10194-012-0460-7] - DOI - PMC - PubMed
BNF 2013
-
- British National Formulary. http://www.medicinescomplete.com/mc/bnf/current/PHP2854‐sumatriptan.htm (Accessed 30 October 2013). London: BMJ Group and Pharmaceutical Press, 2013; Vol. October.
Brandes 2009
-
- Brandes JL, Cady RK, Freitag FG, Smith TR, Chandler P, Fox AW, et al. Needle‐free subcutaneous sumatriptan (Sumavel DosePro): bioequivalence and ease of use. Headache 2009;49(10):1435‐44. - PubMed
Buse 2011
Cook 1995
de Craen 2000
-
- Craen AJ, Tijssen JG, Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. Journal of Neurology 2000;247(3):183‐8. - PubMed
Diamond 2007
-
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] - DOI - PubMed
Diener 2008
-
- Diener HC, Schorn CF, Bingel U, Dodick DW. The importance of placebo in headache research. Cephalalgia 2008;28(10):1003‐11. - PubMed
Ferrari 2002
Gendolla 2008
-
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28(Suppl 2):28‐35. - PubMed
Goadsby 2007
Hazard 2009
IHS 2000
-
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. - PubMed
IHS 2013
Johnston 2010
Law 2013
Leonardi 2005
Linde 2012
Lipton 1999
-
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39(Suppl 2):S20‐S26.
Lipton 2007
-
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. - PubMed
Lucas 2006
Macedo 2006
-
- Macedo A, Farré M, Baños JE. A meta‐analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. European Journal of Clinical Pharmacology 2006;62(3):161‐72. - PubMed
McCrory 2003
Moore 1998
-
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. - PubMed
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5]
Moore 2011
-
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: Examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. - PubMed
Moore 2012
Moore 2013
Munakata 2009
Nuesch 2010
Oldman 2002
-
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. - PubMed
Pascual 2002
PCA 2013
-
- Prescribing and Primary Care team, Health and Social Care Information Centre. Prescription cost analysis, England 2012. http://data.gov.uk/dataset/prescription_cost_analysis_england [Accessed 17 Apr 2013]. Government Statistical Service: Health and Social Care Information Centre, 2013. [ISBN: 978‐1‐84‐636859‐2]
Pierce 2009
-
- Pierce M, Marbury T, O'Neill C, Siegel S, Du W, Sebree T. Zelrix: a novel transdermal formulation of sumatriptan. Headache 2009;49(6):817‐25. - PubMed
Radtke 2009
Shea 2007
Steiner 2013
Stovner 2010
Tfelt‐Hansen 2012
Thornton 2000
Victor 2010
Vos 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

