Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury
- PMID: 24865512
- PMCID: PMC9420084
- DOI: 10.1007/s12035-014-8752-3
Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury
Abstract
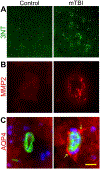
Traumatic brain injury (TBI) is a major cause of death in the young age group and leads to persisting neurological impairment in many of its victims. It may result in permanent functional deficits because of both primary and secondary damages. This review addresses the role of oxidative stress in TBI-mediated secondary damages by affecting the function of the vascular unit, changes in blood-brain barrier (BBB) permeability, posttraumatic edema formation, and modulation of various pathophysiological factors such as inflammatory factors and enzymes associated with trauma. Oxidative stress plays a major role in many pathophysiologic changes that occur after TBI. In fact, oxidative stress occurs when there is an impairment or inability to balance antioxidant production with reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels. ROS directly downregulate proteins of tight junctions and indirectly activate matrix metalloproteinases (MMPs) that contribute to open the BBB. Loosening of the vasculature and perivascular unit by oxidative stress-induced activation of MMPs and fluid channel aquaporins promotes vascular or cellular fluid edema, enhances leakiness of the BBB, and leads to progression of neuroinflammation. Likewise, oxidative stress activates directly the inflammatory cytokines and growth factors such as IL-1β, tumor necrosis factor-α (TNF-α), and transforming growth factor-beta (TGF-β) or indirectly by activating MMPs. In another pathway, oxidative stress-induced degradation of endothelial vascular endothelial growth factor receptor-2 (VEGFR-2) by MMPs leads to a subsequent elevation of cellular/serum VEGF level. The decrease in VEGFR-2 with a subsequent increase in VEGF-A level leads to apoptosis and neuroinflammation via the activation of caspase-1/3 and IL-1β release.
Conflict of interest statement
Figures

References
-
- Lee M, Longoria R, Wilson D (1997) Ballistic waves in high-speed water entry. Fluid Struct 11(7):819–844
-
- Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2(7872):81–84 - PubMed
-
- Gaetz M (2004) The neurophysiology of brain injury. Clin Neurophysiol 115(1):4–18 - PubMed
-
- Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7(8):728–741 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

