Synthesis of novel lipophilic N-substituted norcantharimide derivatives and evaluation of their anticancer activities
- PMID: 24865603
- PMCID: PMC6271113
- DOI: 10.3390/molecules19066911
Synthesis of novel lipophilic N-substituted norcantharimide derivatives and evaluation of their anticancer activities
Erratum in
-
Correction: Wu et al. Synthesis of Novel Lipophilic N-Substituted Norcantharimide Derivatives and Evaluation of Their Anticancer Activities. Molecules 2014, 19, 6911-6928.Molecules. 2025 May 22;30(11):2256. doi: 10.3390/molecules30112256. Molecules. 2025. PMID: 40492287 Free PMC article.
Abstract
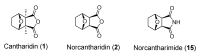
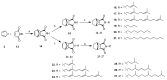
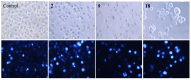
This research attempted to study the effect of lipophilicity on the anticancer activity of N-substituted norcantharimide derivatives. Twenty-three compounds were synthesized and their cytotoxicities against five human cancer cell lines studied. The lipophilicity of each derivative was altered by its substituent, an alkyl, alkyloxy, terpenyl or terpenyloxy group at the N-position of norcantharimide. Further, among all synthesized derivatives studied, the compounds N-farnesyloxy-7-oxabicyclo[2.2.1]heptane-2,3-dicarboximide (9), and N-farnesyl-7-oxabicyclo[2.2.1]heptane-2,3-dicarboximide (18), have shown the highest cytotoxicity, anti-proliferative and apoptotic effect against human liver carcinoma HepG2 cell lines, yet displayed no significant cytotoxic effect on normal murine embryonic liver BNL CL.2 cells. Their overall performance led us to believe that these two compounds might be potential candidates for anticancer drugs development.
Figures
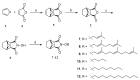

Similar articles
-
Anticancer Potential of Halogen Derivatives of Methyl 6-Acetyl-5-Hydroxy-2-Methyl-1-Benzofuran-3-Carboxylate.Int J Mol Sci. 2025 Jun 8;26(12):5493. doi: 10.3390/ijms26125493. Int J Mol Sci. 2025. PMID: 40564956 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Biological and In Silico Studies of a Novel Pyrazolo[3,4-d]Thiazole Derivatives: Anticancer, Anti-Inflammatory, Molecular Docking, and SAR.Chem Biodivers. 2025 Jun;22(6):e202403173. doi: 10.1002/cbdv.202403173. Epub 2025 Feb 26. Chem Biodivers. 2025. PMID: 39921917
-
Design, synthesis, and biological evaluation of N-(2-amino-phenyl)-5-(4-aryl- pyrimidin-2-yl) amino)-1H-indole-2-carboxamide derivatives as novel inhibitors of CDK9 and class I HDACs for cancer treatment.Bioorg Chem. 2025 Jul 15;162:108577. doi: 10.1016/j.bioorg.2025.108577. Epub 2025 May 10. Bioorg Chem. 2025. PMID: 40383016
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
Cited by
-
Synthesis and Biological Evaluation of Lipophilic 1,4-Naphthoquinone Derivatives against Human Cancer Cell Lines.Molecules. 2015 Jun 30;20(7):11994-2015. doi: 10.3390/molecules200711994. Molecules. 2015. PMID: 26133763 Free PMC article.
-
Comparative assessment of therapeutic safety of norcantharidin, N-farnesyloxy-norcantharimide, and N-farnesyl-norcantharimide against Jurkat T cells relative to human normal lymphoblast: A quantitative pilot study.Medicine (Baltimore). 2016 Aug;95(31):e4467. doi: 10.1097/MD.0000000000004467. Medicine (Baltimore). 2016. PMID: 27495082 Free PMC article.
-
Insects: an underrepresented resource for the discovery of biologically active natural products.Acta Pharm Sin B. 2017 Jul;7(4):409-426. doi: 10.1016/j.apsb.2017.05.001. Epub 2017 May 30. Acta Pharm Sin B. 2017. PMID: 28752026 Free PMC article. Review.
-
N-Farnesyloxy-norcantharimide inhibits progression of human leukemic Jurkat T cells through regulation of mitogen-activated protein kinase and interleukin-2 production.Anticancer Drugs. 2015 Nov;26(10):1034-42. doi: 10.1097/CAD.0000000000000284. Anticancer Drugs. 2015. PMID: 26288134 Free PMC article.
-
Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin.Molecules. 2022 Nov 10;27(22):7740. doi: 10.3390/molecules27227740. Molecules. 2022. PMID: 36431851 Free PMC article. Review.
References
-
- Karras D.J., Farrell S.E., Harrigan R.A., Henretig F.M., Gealt L. Poisoning from “Spanish fly” (Cantharidin) Am. J. Emerg. Med. 1996;14:478–483. - PubMed
-
- Chen R.T., Hua Z., Yang J.L., Han J.X., Zhang S.Y., Lü F.L., Xü B. Studies on antitumor actions of cantharidin. Chin. Med. J. 1980;93:183–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

