Flos Puerariae extract prevents myocardial apoptosis via attenuation oxidative stress in streptozotocin-induced diabetic mice
- PMID: 24865768
- PMCID: PMC4035321
- DOI: 10.1371/journal.pone.0098044
Flos Puerariae extract prevents myocardial apoptosis via attenuation oxidative stress in streptozotocin-induced diabetic mice
Abstract
Background: Diabetic cardiomyopathy (DCM) suggests a direct cellular insult to myocardium. Apoptosis is considered as one of the hallmarks of DCM. Oxidative stress plays a key role in the pathogenesis of DCM. In this study, we explored the prevention of myocardial apoptosis by crude extract from Flos Puerariae (FPE) in experimental diabetic mice.
Methods: Experimental diabetic model was induced by intraperitoneally injection of streptozotocin (STZ, 50 mg/kg/day) for five consecutive days in C57BL/6J mice. FPE (100, 200 mg/kg) was orally administrated once a day for ten weeks. Cardiac structure changes, apoptosis, superoxide production, NADPH oxidase subunits expression (gp91phox, p47phox, and p67phox), and related regulatory factors were assessed in the heart of mice.
Results: Diabetic mice were characterized by high blood glucose (≥11.1 mmol/L) and reduced body weight. In the end of the experiment, aberrant myofilament structure, as well as TUNEL positive cardiac cells coupled with increased Bax/Bcl-2 ratio and Caspase-3 expression was found in diabetic mice. Moreover, ROS formation, the ratio of NADP+/NADPH and NADPH oxidase subunits expression of gp91phox and p47phox, lipid peroxidation level was significantly increased, while antioxidant enzyme SOD and GSH-Px activity were reduced in the myocardial tissue of diabetic mice. In contrast, treatment with FPE resulted in a normalized glucose and weight profile. FPE administration also preserved myocardial structure and reduced apoptotic cardiac cell death in diabetic mice. The elevated markers of oxidative stress were significantly reversed by FPE supplementation. Further, FPE treatment markedly inhibited the increased Bax/Bcl-2 ratio and Caspase-3 expression, as well as suppressed JNK and P38 MAPK activation in the heart of diabetic mice.
Conclusions: Our data demonstrate for the first time that FPE may have therapeutic potential for STZ-induced diabetic cardiomyopathy through preventing myocardial apoptosis via attenuation oxidative stress. And this effect is probably mediated by JNK and P38 MAPK signaling pathway.
Conflict of interest statement
Figures

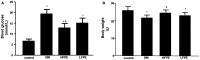





Similar articles
-
Azelnidipine prevents cardiac dysfunction in streptozotocin-diabetic rats by reducing intracellular calcium accumulation, oxidative stress and apoptosis.Cardiovasc Diabetol. 2011 Nov 4;10:97. doi: 10.1186/1475-2840-10-97. Cardiovasc Diabetol. 2011. PMID: 22054019 Free PMC article.
-
Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis.Food Chem Toxicol. 2014 Jan;63:221-32. doi: 10.1016/j.fct.2013.11.013. Epub 2013 Nov 20. Food Chem Toxicol. 2014. PMID: 24269735
-
Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats.PLoS One. 2012;7(12):e52013. doi: 10.1371/journal.pone.0052013. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251674 Free PMC article.
-
l-Arginine: A multifaceted regulator of diabetic cardiomyopathy.Biochem Biophys Res Commun. 2025 May 1;761:151720. doi: 10.1016/j.bbrc.2025.151720. Epub 2025 Mar 28. Biochem Biophys Res Commun. 2025. PMID: 40186920 Review.
-
Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy.Oxid Med Cell Longev. 2022 Jan 22;2022:5913374. doi: 10.1155/2022/5913374. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35103095 Free PMC article. Review.
Cited by
-
The Role of p38 MAPK in the Development of Diabetic Cardiomyopathy.Int J Mol Sci. 2016 Jun 30;17(7):1037. doi: 10.3390/ijms17071037. Int J Mol Sci. 2016. PMID: 27376265 Free PMC article. Review.
-
Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway.Int J Mol Med. 2018 Apr;41(4):1867-1876. doi: 10.3892/ijmm.2018.3419. Epub 2018 Jan 23. Int J Mol Med. 2018. PMID: 29393353 Free PMC article.
-
Single-cell RNA-seq of heart reveals intercellular communication drivers of myocardial fibrosis in diabetic cardiomyopathy.Elife. 2023 Apr 3;12:e80479. doi: 10.7554/eLife.80479. Elife. 2023. PMID: 37010266 Free PMC article.
-
Antioxidative Effects of Natural Products on Diabetic Cardiomyopathy.J Diabetes Res. 2017;2017:2070178. doi: 10.1155/2017/2070178. Epub 2017 Oct 18. J Diabetes Res. 2017. PMID: 29181412 Free PMC article. Review.
-
Roles and Mechanisms of Herbal Medicine for Diabetic Cardiomyopathy: Current Status and Perspective.Oxid Med Cell Longev. 2017;2017:8214541. doi: 10.1155/2017/8214541. Epub 2017 Oct 24. Oxid Med Cell Longev. 2017. PMID: 29204251 Free PMC article. Review.
References
-
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, et al. (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30: 595–602. - PubMed
-
- Ho FM, Liu SH, Liau CS, Huang PJ, Lin-Shiau SY (2000) High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation 101: 2618–2624. - PubMed
-
- Cai L, Wang Y, Zhou G, Chen T, Song Y, et al. (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48: 1688–1697. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

