Research designs for proof-of-concept chronic pain clinical trials: IMMPACT recommendations
- PMID: 24865794
- PMCID: PMC4500524
- DOI: 10.1016/j.pain.2014.05.025
Research designs for proof-of-concept chronic pain clinical trials: IMMPACT recommendations
Abstract
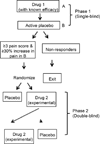
Proof-of-concept (POC) clinical trials play an important role in developing novel treatments and determining whether existing treatments may be efficacious in broader populations of patients. The goal of most POC trials is to determine whether a treatment is likely to be efficacious for a given indication and thus whether it is worth investing the financial resources and participant exposure necessary for a confirmatory trial of that intervention. A challenge in designing POC trials is obtaining sufficient information to make this important go/no-go decision in a cost-effective manner. An IMMPACT consensus meeting was convened to discuss design considerations for POC trials in analgesia, with a focus on maximizing power with limited resources and participants. We present general design aspects to consider including patient population, active comparators and placebos, study power, pharmacokinetic-pharmacodynamic relationships, and minimization of missing data. Efficiency of single-dose studies for treatments with rapid onset is discussed. The trade-off between parallel-group and crossover designs with respect to overall sample sizes, trial duration, and applicability is summarized. The advantages and disadvantages of more recent trial designs, including N-of-1 designs, enriched designs, adaptive designs, and sequential parallel comparison designs, are summarized, and recommendations for consideration are provided. More attention to identifying efficient yet powerful designs for POC clinical trials of chronic pain treatments may increase the percentage of truly efficacious pain treatments that are advanced to confirmatory trials while decreasing the percentage of ineffective treatments that continue to be evaluated rather than abandoned.
Keywords: Clinical trials; IMMPACT; Methodology; Proof of concept.
Copyright © 2014 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest related to the issues discussed in this manuscript.
Figures


References
-
- Andre-Obadia N, Magnin M, Garcia-Larrea L. On the importance of placebo timing in rTMS studies for pain relief. Pain. 2011;152:1233–1237. - PubMed
-
- Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88. - PubMed
-
- Backonja MM, Attal N, Baron R, Bouhassira D, Drangholt M, Dyck PJ, Edwards RR, Freeman R, Gracely R, Haanpää MH, Hansson P, Hatem SM, Krumova EK, Jensen TS, Maier C, Mick G, Rice AS, Rolke R, Treede RD, Serra J, Toelle T, Tugnoli V, Walk D, Walalce MS, Ware M, Yarnitsky D, Ziegler D. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG concensus. Pain. 2013;154:1807–1819. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

