Impairments of hepatic gluconeogenesis and ketogenesis in PPARα-deficient neonatal mice
- PMID: 24865983
- PMCID: PMC4101633
- DOI: 10.1152/ajpendo.00087.2014
Impairments of hepatic gluconeogenesis and ketogenesis in PPARα-deficient neonatal mice
Abstract
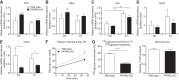
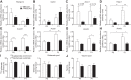
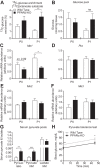
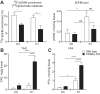
Peroxisome proliferator activated receptor-α (PPARα) is a master transcriptional regulator of hepatic metabolism and mediates the adaptive response to fasting. Here, we demonstrate the roles for PPARα in hepatic metabolic adaptations to birth. Like fasting, nutrient supply is abruptly altered at birth when a transplacental source of carbohydrates is replaced by a high-fat, low-carbohydrate milk diet. PPARα-knockout (KO) neonatal mice exhibit relative hypoglycemia due to impaired conversion of glycerol to glucose. Although hepatic expression of fatty acyl-CoA dehydrogenases is imparied in PPARα neonates, these animals exhibit normal blood acylcarnitine profiles. Furthermore, quantitative metabolic fate mapping of the medium-chain fatty acid [(13)C]octanoate in neonatal mouse livers revealed normal contribution of this fatty acid to the hepatic TCA cycle. Interestingly, octanoate-derived carbon labeled glucose uniquely in livers of PPARα-KO neonates. Relative hypoketonemia in newborn PPARα-KO animals could be mechanistically linked to a 50% decrease in de novo hepatic ketogenesis from labeled octanoate. Decreased ketogenesis was associated with diminished mRNA and protein abundance of the fate-committing ketogenic enzyme mitochondrial 3-hydroxymethylglutaryl-CoA synthase (HMGCS2) and decreased protein abundance of the ketogenic enzyme β-hydroxybutyrate dehydrogenase 1 (BDH1). Finally, hepatic triglyceride and free fatty acid concentrations were increased 6.9- and 2.7-fold, respectively, in suckling PPARα-KO neonates. Together, these findings indicate a primary defect of gluconeogenesis from glycerol and an important role for PPARα-dependent ketogenesis in the disposal of hepatic fatty acids during the neonatal period.
Keywords: 3-hydroxymethylglutaryl-CoA synthase; glucose homeostasis; ketone body metabolism; metabolic adaptation to birth; nuclear magnetic resonance substrate fate mapping; peroxisome proliferator-activated receptor-α.
Copyright © 2014 the American Physiological Society.
Figures


Similar articles
-
Impact of peripheral ketolytic deficiency on hepatic ketogenesis and gluconeogenesis during the transition to birth.J Biol Chem. 2013 Jul 5;288(27):19739-49. doi: 10.1074/jbc.M113.454868. Epub 2013 May 20. J Biol Chem. 2013. PMID: 23689508 Free PMC article.
-
Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting.J Biol Chem. 2000 Sep 15;275(37):28918-28. doi: 10.1074/jbc.M910350199. J Biol Chem. 2000. PMID: 10844002
-
The role of chicken ovalbumin upstream promoter transcription factor II in the regulation of hepatic fatty acid oxidation and gluconeogenesis in newborn mice.Am J Physiol Endocrinol Metab. 2015 May 15;308(10):E868-78. doi: 10.1152/ajpendo.00433.2014. Epub 2015 Mar 17. Am J Physiol Endocrinol Metab. 2015. PMID: 25783893
-
Metabolic adaptations to change of nutrition at birth.Biol Neonate. 1990;58 Suppl 1:3-15. doi: 10.1159/000243294. Biol Neonate. 1990. PMID: 2265217 Review.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
Cited by
-
Hepatocyte-specific deletion of Pparα promotes NAFLD in the context of obesity.Sci Rep. 2020 Apr 16;10(1):6489. doi: 10.1038/s41598-020-63579-3. Sci Rep. 2020. PMID: 32300166 Free PMC article.
-
CDKN2A/p16INK4a suppresses hepatic fatty acid oxidation through the AMPKα2-SIRT1-PPARα signaling pathway.J Biol Chem. 2020 Dec 11;295(50):17310-17322. doi: 10.1074/jbc.RA120.012543. Epub 2020 Oct 9. J Biol Chem. 2020. PMID: 33037071 Free PMC article.
-
Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice.Nutr Metab (Lond). 2016 Sep 26;13:62. doi: 10.1186/s12986-016-0122-x. eCollection 2016. Nutr Metab (Lond). 2016. PMID: 27708682 Free PMC article.
-
β-Hydroxybutyrate is reduced in humans with obesity-related NAFLD and displays a dose-dependent effect on skeletal muscle mitochondrial respiration in vitro.Am J Physiol Endocrinol Metab. 2020 Jul 1;319(1):E187-E195. doi: 10.1152/ajpendo.00058.2020. Epub 2020 May 12. Am J Physiol Endocrinol Metab. 2020. PMID: 32396388 Free PMC article.
-
Role of hepatocyte RIPK1 in maintaining liver homeostasis during metabolic challenges.Elife. 2025 Jan 31;13:RP96798. doi: 10.7554/eLife.96798. Elife. 2025. PMID: 39886919 Free PMC article.
References
-
- Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 10: 330–344, 2013 - PubMed
-
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

