Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging
- PMID: 24865996
- PMCID: PMC4126103
- DOI: 10.1038/jcbfm.2014.97
Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging
Abstract
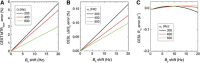
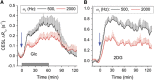
Uptake of administered D-glucose (Glc) or 2-deoxy-D-glucose (2DG) has been indirectly mapped through the chemical exchange (CE) between glucose hydroxyl and water protons using CE-dependent saturation transfer (glucoCEST) magnetic resonance imaging (MRI). We propose an alternative technique-on-resonance CE-sensitive spin-lock (CESL) MRI-to enhance responses to glucose changes. Phantom data and simulations suggest higher sensitivity for this 'glucoCESL' technique (versus glucoCEST) in the intermediate CE regime relevant to glucose. Simulations of CESL signals also show insensitivity to B0-fluctuations. Several findings are apparent from in vivo glucoCESL studies of rat brain at 9.4 Tesla with intravenous injections. First, dose-dependent responses are nearly linearly for 0.25-, 0.5-, and 1-g/kg Glc administration (obtained with 12-second temporal resolution), with changes robustly detected for all doses. Second, responses at a matched dose of 1 g/kg are much larger and persist for a longer duration for 2DG versus Glc administration, and are minimal for mannitol as an osmolality control. And third, with similar increases in steady-state blood glucose levels, glucoCESL responses are ∼2.2 times higher for 2DG versus Glc, consistent with their different metabolic properties. Overall, we show that glucoCESL MRI could be a highly sensitive and quantifiable tool for glucose transport and metabolism studies.
Figures




References
-
- Choi I-Y, Lee S-P, Kim S-G, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menton model and simultaneous measurements of cerebral blood flow changes during hyperglycemia. J Cereb Blood Flow Metab. 2001;21:653–663. - PubMed
-
- Choi IY, Lei HX, Gruetter R. Effect of deep pentobarbital anesthesia on neurotransmitter metabolism in vivo: on the correlation of total glucose consumption with glutamatergic action. J Cereb Blood Flow Metab. 2002;22:1343–1351. - PubMed
-
- de Graaf RA, Pan JW, Telang F, Lee JH, Brown P, Novotny EJ, et al. Differentiation of glucose transport in human brain gray and white matter. J Cereb Blood Flow Metab. 2001;21:483–492. - PubMed
-
- Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

