Differential methylation in CN-AML preferentially targets non-CGI regions and is dictated by DNMT3A mutational status and associated with predominant hypomethylation of HOX genes
- PMID: 24866170
- PMCID: PMC4164496
- DOI: 10.4161/epi.29315
Differential methylation in CN-AML preferentially targets non-CGI regions and is dictated by DNMT3A mutational status and associated with predominant hypomethylation of HOX genes
Abstract
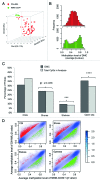
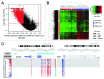
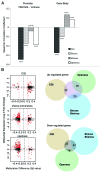
The extent and role of aberrant DNA methylation in promoter CpG islands (CGIs) have been extensively studied in leukemia and other malignancies. Still, CGIs represent only a small fraction of the methylome. We aimed to characterize genome-wide differential methylation of cytogenetically normal AML (CN-AML) cells compared with normal CD34(+) bone marrow cells using the Illumina 450K methylation array. Differential methylation in CN-AML was most prominent in genomic areas far from CGIs, in so called open sea regions. Furthermore, differential methylation was specifically found in genes encoding transcription factors (TFs), with WT1 being the most differentially methylated TF. Among genetic mutations in AML, DNMT3A mutations showed the most prominent association with the DNA methylation pattern, characterized by hypomethylation of CGIs (as compared with DNMT3A wild type cases). The differential methylation in DNMT3A mutant cells vs. wild type cells was predominantly found in HOX genes, which were hypomethylated. These results were confirmed and validated in an independent CN-AML cohort. In conclusion, we show that, in CN-AML, the most pronounced changes in DNA methylation occur in non-CGI regions and that DNMT3A mutations confer a pattern of global hypomethylation that specifically targets HOX genes.
Keywords: DNA methylation; DNMT3A; Homeobox gene family; acute myeloid leukemia; non-CGI region.
Figures


References
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–51. doi: 10.1182/blood-2009-03-209262. - DOI - PubMed
-
- Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, Thomas X, Raffoux E, Lamandin C, Castaigne S, et al. ALFA Group Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: a study from the Acute Leukemia French Association (ALFA) Blood. 2002;100:2717–23. doi: 10.1182/blood-2002-03-0990. - DOI - PubMed
-
- Schlenk RF, Döhner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, Habdank M, Späth D, Morgan M, Benner A, et al. German-Austrian Acute Myeloid Leukemia Study Group Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
