The amygdala mediates the emotional modulation of threat-elicited skin conductance response
- PMID: 24866521
- PMCID: PMC4115032
- DOI: 10.1037/a0036636
The amygdala mediates the emotional modulation of threat-elicited skin conductance response
Abstract
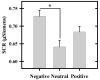
The ability to respond adaptively to threats in a changing environment is an important emotional function. The amygdala is a critical component of the neural circuit that mediates many emotion-related processes, and thus likely plays an important role in modulating the peripheral emotional response to threat. However, prior research has largely focused on the amygdala's response to stimuli that signal impending threat, giving less attention to the amygdala's response to the threat itself. From a functional perspective, however, it is the response to the threat itself that is most biologically relevant. Thus, understanding the factors that influence the amygdala's response to threat is critical for a complete understanding of adaptive emotional processes. Therefore, we used functional MRI to investigate factors (i.e., valence and arousal of co-occurring visual stimuli) that influence the amygdala's response to threat (loud white noise). We also assessed whether changes in amygdala activity varied with the peripheral expression of emotion (indexed via skin conductance response; SCR). The results showed that threat-elicited amygdala activation varied with the arousal, not valence, of emotional images. More specifically, threat-elicited amygdala activation was larger to the threat when presented during high-arousal (i.e., negative and positive) versus low-arousal (i.e., neutral) images. Further, the threat-elicited amygdala response was positively correlated with threat-elicited SCR. These findings indicate the amygdala's response to threat is modified by the nature (e.g., arousal) of other stimuli in the environment. In turn, the amygdala appears to mediate important aspects of the peripheral emotional response to threat.
Figures

Similar articles
-
Pavlovian conditioned diminution of the neurobehavioral response to threat.Neurosci Biobehav Rev. 2018 Jan;84:218-224. doi: 10.1016/j.neubiorev.2017.11.021. Epub 2017 Dec 2. Neurosci Biobehav Rev. 2018. PMID: 29203422 Free PMC article. Review.
-
Amygdala responses to Valence and its interaction by arousal revealed by MEG.Int J Psychophysiol. 2014 Jul;93(1):121-33. doi: 10.1016/j.ijpsycho.2013.05.006. Epub 2013 May 18. Int J Psychophysiol. 2014. PMID: 23688672
-
Amygdala response to both positively and negatively valenced stimuli.Neuroreport. 2001 Aug 28;12(12):2779-83. doi: 10.1097/00001756-200108280-00036. Neuroreport. 2001. PMID: 11522965 Clinical Trial.
-
The role of the human amygdala in the production of conditioned fear responses.Neuroimage. 2005 Jul 15;26(4):1193-200. doi: 10.1016/j.neuroimage.2005.03.020. Epub 2005 Apr 20. Neuroimage. 2005. PMID: 15961053 Clinical Trial.
-
Fear, faces, and the human amygdala.Curr Opin Neurobiol. 2008 Apr;18(2):166-72. doi: 10.1016/j.conb.2008.06.006. Epub 2008 Aug 12. Curr Opin Neurobiol. 2008. PMID: 18655833 Free PMC article. Review.
Cited by
-
Affective state and locus of control modulate the neural response to threat.Neuroimage. 2015 Nov 1;121:217-26. doi: 10.1016/j.neuroimage.2015.07.034. Epub 2015 Jul 18. Neuroimage. 2015. PMID: 26196669 Free PMC article.
-
Controllability modulates the neural response to predictable but not unpredictable threat in humans.Neuroimage. 2015 Oct 1;119:371-81. doi: 10.1016/j.neuroimage.2015.06.086. Epub 2015 Jul 3. Neuroimage. 2015. PMID: 26149610 Free PMC article.
-
Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress.Behav Neurosci. 2019 Apr;133(2):203-211. doi: 10.1037/bne0000305. Behav Neurosci. 2019. PMID: 30907618 Free PMC article.
-
Parental Reactivity to Disruptive Behavior in Toddlerhood: An Experimental Study.J Abnorm Child Psychol. 2019 May;47(5):779-790. doi: 10.1007/s10802-018-0489-4. J Abnorm Child Psychol. 2019. PMID: 30370463 Free PMC article.
-
Pavlovian conditioned diminution of the neurobehavioral response to threat.Neurosci Biobehav Rev. 2018 Jan;84:218-224. doi: 10.1016/j.neubiorev.2017.11.021. Epub 2017 Dec 2. Neurosci Biobehav Rev. 2018. PMID: 29203422 Free PMC article. Review.
References
-
- Bradley MM, Cuthbert BN, Lang PJ. Startle and emotion: lateral acoustic probes and the bilateral blink. Psychophysiology. 1991;28(3):285–95. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1946894. - PubMed
-
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8938120. - PubMed
-
- Brühl AB, Rufer M, Delsignore A, Kaffenberger T, Jäncke L, Herwig U. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Research. 2011;1378:72–83. doi:10.1016/j.brainres.2010.12.084. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

