The psmα locus regulates production of Staphylococcus aureus alpha-toxin during infection
- PMID: 24866799
- PMCID: PMC4136214
- DOI: 10.1128/IAI.00089-14
The psmα locus regulates production of Staphylococcus aureus alpha-toxin during infection
Abstract
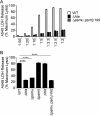
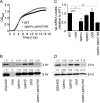
Staphylococcus aureus is a leading cause of human bacterial infection, causing a wide spectrum of disease ranging from skin and soft tissue infections to life-threatening pneumonia and sepsis. S. aureus toxins play an essential role in disease pathogenesis, contributing to both immunomodulation and host tissue injury. Prominent among these toxins are the membrane-active pore-forming cytolysin alpha-toxin (Hla) and the amphipathic α-helical phenol-soluble modulin (PSM) peptides. As deletion of either the hla or psm locus leads to a phenotypically similar virulence defect in skin and soft tissue infection, we sought to determine the relative contribution of each locus to disease pathogenesis. Here we show that production of Hla can be modulated by PSM expression. An S. aureus mutant lacking PSM expression exhibits a transcriptional delay in hla mRNA production and therefore fails to secrete normal levels of Hla at early phases of growth. This leads to attenuation of virulence in vitro and in murine skin and lung models of infection, correlating with reduced recovery of Hla from host tissues. Production of Hla and restoration of staphylococcal virulence can be achieved in the psm mutant by plasmid-driven overexpression of hla. Our study suggests the coordinated action of Hla and PSMs in host tissue during early pathogenesis, confirming a major role for Hla in epithelial injury during S. aureus infection. These findings highlight the possibility that therapeutics targeting PSM production may simultaneously prevent Hla-mediated tissue injury.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Jacobson C, Smulders M, Gemmen E, Bharmal M. 2007. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998-2003). Clin. Infect. Dis. 45:1132–1140. 10.1086/522186. - DOI - PubMed
-
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

