A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro
- PMID: 24867320
- PMCID: PMC4035295
- DOI: 10.1371/journal.pone.0097864
A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro
Erratum in
-
A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro.PLoS One. 2014 Jul 3;9(7):e101859. doi: 10.1371/journal.pone.0101859. eCollection 2014. PLoS One. 2014. PMID: 24991987 Free PMC article.
Abstract
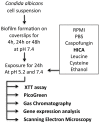
The ability of C. albicans to form biofilms is a major virulence factor and a challenge for management. This is evident in biofilm-associated chronic oral-oesophageal candidosis, which has been shown to be potentially carcinogenic in vivo. We have previously shown that most Candida spp. can produce significant levels of mutagenic acetaldehyde (ACH). ACH is also an important mediator of candidal biofilm formation. We have also reported that D,L-2-hydroxyisocaproic acid (HICA) significantly inhibits planktonic growth of C. albicans. The aim of the present study was to investigate the effect of HICA on C. albicans biofilm formation and ACH production in vitro. Inhibition of biofilm formation by HICA, analogous control compounds or caspofungin was measured using XTT to measure biofilm metabolic activity and PicoGreen as a marker of biomass. Biofilms were visualised by scanning electron microscopy (SEM). ACH levels were measured by gas chromatography. Transcriptional changes in the genes involved in ACH metabolism were measured using RT-qPCR. The mean metabolic activity and biomass of all pre-grown (4, 24, 48 h) biofilms were significantly reduced after exposure to HICA (p<0.05) with the largest reductions seen at acidic pH. Caspofungin was mainly active against biofilms pre-grown for 4 h at neutral pH. Mutagenic levels (>40 µM) of ACH were detected in 24 and 48 h biofilms at both pHs. Interestingly, no ACH production was detected from D-glucose in the presence of HICA at acidic pH (p<0.05). Expression of genes responsible for ACH catabolism was up-regulated by HICA but down-regulated by caspofungin. SEM showed aberrant hyphae and collapsed hyphal structures during incubation with HICA at acidic pH. We conclude that HICA has potential as an antifungal agent with ability to inhibit C. albicans cell growth and biofilm formation. HICA also significantly reduces the mutagenic potential of C. albicans biofilms, which may be important when treating bacterial-fungal biofilm infections.
Conflict of interest statement
Figures

Similar articles
-
DL-2-hydroxyisocaproic acid attenuates inflammatory responses in a murine Candida albicans biofilm model.Clin Vaccine Immunol. 2014 Sep;21(9):1240-5. doi: 10.1128/CVI.00339-14. Epub 2014 Jul 2. Clin Vaccine Immunol. 2014. PMID: 24990903 Free PMC article.
-
A novel antifungal is active against Candida albicans biofilms and inhibits mutagenic acetaldehyde production in vitro.PLoS One. 2014 Jul 3;9(7):e101859. doi: 10.1371/journal.pone.0101859. eCollection 2014. PLoS One. 2014. PMID: 24991987 Free PMC article.
-
Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis.J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. J Mycol Med. 2020. PMID: 32008964
-
Antifungal susceptibility of Candida albicans in biofilms.Mycoses. 2012 May;55(3):199-204. doi: 10.1111/j.1439-0507.2011.02076.x. Epub 2011 Jul 28. Mycoses. 2012. PMID: 21793943 Review.
-
Biofilm of Candida albicans: formation, regulation and resistance.J Appl Microbiol. 2021 Jul;131(1):11-22. doi: 10.1111/jam.14949. Epub 2020 Dec 9. J Appl Microbiol. 2021. PMID: 33249681 Review.
Cited by
-
Influence of culture media on biofilm formation by Candida species and response of sessile cells to antifungals and oxidative stress.Biomed Res Int. 2015;2015:783639. doi: 10.1155/2015/783639. Epub 2015 Feb 1. Biomed Res Int. 2015. PMID: 25705688 Free PMC article.
-
Alteration of Fermentative Metabolism Enhances Mucor circinelloides Virulence.Infect Immun. 2020 Jan 22;88(2):e00434-19. doi: 10.1128/IAI.00434-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31685547 Free PMC article.
-
Two promising Bacillus-derived antifungal lipopeptide leads AF4 and AF5 and their combined effect with fluconazole on the in vitro Candida glabrata biofilms.Front Pharmacol. 2024 Apr 19;15:1334419. doi: 10.3389/fphar.2024.1334419. eCollection 2024. Front Pharmacol. 2024. PMID: 38708082 Free PMC article.
-
Local Acetaldehyde-An Essential Role in Alcohol-Related Upper Gastrointestinal Tract Carcinogenesis.Cancers (Basel). 2018 Jan 5;10(1):11. doi: 10.3390/cancers10010011. Cancers (Basel). 2018. PMID: 29303995 Free PMC article. Review.
-
DL-2-hydroxyisocaproic acid attenuates inflammatory responses in a murine Candida albicans biofilm model.Clin Vaccine Immunol. 2014 Sep;21(9):1240-5. doi: 10.1128/CVI.00339-14. Epub 2014 Jul 2. Clin Vaccine Immunol. 2014. PMID: 24990903 Free PMC article.
References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, et al. (2012) Hidden killers: human fungal infections. Sci Transl Med 4 165rv113 - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322. - PubMed
-
- Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35: 340–355. - PubMed
-
- Baillie GS, Douglas LJ (1999) Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol 310: 644–656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

