Long-term safety of perampanel and seizure outcomes in refractory partial-onset seizures and secondarily generalized seizures: results from phase III extension study 307
- PMID: 24867391
- PMCID: PMC4283992
- DOI: 10.1111/epi.12643
Long-term safety of perampanel and seizure outcomes in refractory partial-onset seizures and secondarily generalized seizures: results from phase III extension study 307
Abstract
Objective: To evaluate safety, tolerability, seizure frequency, and regional variations in treatment responses with the AMPA antagonist, perampanel, in a large extension study during up to 3 years of treatment.
Methods: Patients ≥ 12 years old with partial-onset seizures despite treatment with 1-3 antiepileptic drugs at baseline completed a perampanel phase III trial and entered extension study 307 (NCT00735397). Patients were titrated to 12 mg/day (or their individual maximum tolerated dose) during the blinded conversion period, followed by open-label maintenance. Exposure, safety (adverse events [AEs], vital signs, weight, electrocardiography [ECG], laboratory values) and seizure outcomes were analyzed; key measures were assessed by geographic regions.
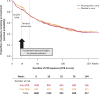
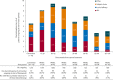
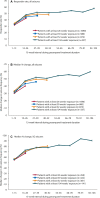
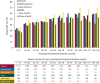
Results: Among 1,216 patients, median exposure was 1.5 years (range 1 week to 3.3 years), with >300 patients treated for >2 years. Treatment retention was 58.5% at cutoff. AEs reported in ≥ 10% of patients were dizziness, somnolence, headache, fatigue, irritability, and weight increase. Only dizziness and irritability caused discontinuation in >1% of patients (3.9% and 1.3%, respectively). The only serious AEs reported in >1% of patients were epilepsy-related (convulsion, 3.0%; status epilepticus, 1.1%). No clinically relevant changes in vital signs, ECG or laboratory parameters were seen. After titration/conversion, responder rate and median percentage change from baseline in seizure frequency were stable: 46% for both measures at 9 months (in 980 patients with ≥ 9 months' exposure) and 58% and 60%, respectively, at 2 years (in the 337 patients with 2 years' exposure). Median percentage reduction in frequency of secondarily generalized (SG) seizures ranged from 77% at 9 months (N = 422) to 90% at 2 years (N = 141). Among the 694 patients with maintenance data ≥ 1 year, 5.3% were seizure-free for the entire year.
Significance: No new safety signals emerged during up to 3 years of perampanel exposure in 39 countries. Seizure responses remained stable, with marked reductions, particularly in SG seizures.
Keywords: AMPA receptor; Antagonist; Antiepilepsy drugs; Epilepsy; Seizure freedom.
© 2014 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures

Similar articles
-
Efficacy and safety of perampanel in patients with drug-resistant partial seizures after conversion from double-blind placebo to open-label perampanel.Epilepsy Res. 2015 Aug;114:131-40. doi: 10.1016/j.eplepsyres.2015.04.011. Epub 2015 May 1. Epilepsy Res. 2015. PMID: 26088896 Clinical Trial.
-
Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open-label extension of phase III randomized trials: Study 307.Epilepsia. 2018 Apr;59(4):866-876. doi: 10.1111/epi.14044. Epub 2018 Mar 25. Epilepsia. 2018. PMID: 29574701 Clinical Trial.
-
Perampanel, a selective, noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307.Epilepsia. 2013 Jan;54(1):126-34. doi: 10.1111/j.1528-1167.2012.03648.x. Epub 2012 Aug 20. Epilepsia. 2013. PMID: 22905878 Clinical Trial.
-
Perampanel: expanding therapeutic options for patients with medically refractory secondary generalized convulsive seizures.Acta Neurol Scand Suppl. 2013;(197):36-43. doi: 10.1111/ane.12103. Acta Neurol Scand Suppl. 2013. PMID: 23480155 Review.
-
Perampanel in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus.Expert Rev Clin Pharmacol. 2015;8(6):733-40. doi: 10.1586/17512433.2015.1091303. Epub 2015 Oct 5. Expert Rev Clin Pharmacol. 2015. PMID: 26436331 Review.
Cited by
-
Evaluation of perampanel as monotherapy for focal seizures: Experience from open-label extension studies.Epilepsy Behav Case Rep. 2017 Dec 1;9:1-5. doi: 10.1016/j.ebcr.2017.11.001. eCollection 2018. Epilepsy Behav Case Rep. 2017. PMID: 29707476 Free PMC article.
-
Bioequivalence study of perampanel oral suspension in healthy Chinese subjects under fasting and fed conditions.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 26. doi: 10.1007/s00210-025-04004-2. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40137967
-
Efficacy and Tolerability of Perampanel in Brain Tumor-Related Epilepsy: A Systematic Review.Biomedicines. 2023 Feb 21;11(3):651. doi: 10.3390/biomedicines11030651. Biomedicines. 2023. PMID: 36979629 Free PMC article. Review.
-
Two Targets Are Better Than One: A New Strategy to Increase the Specificity of Anti-Epileptic Drugs.Epilepsy Curr. 2017 Jul-Aug;17(4):235-236. doi: 10.5698/1535-7597.17.4.235. Epilepsy Curr. 2017. PMID: 29225530 Free PMC article. No abstract available.
-
Effectiveness and safety of perampanel in adults with mesial temporal epilepsy: A single-center postmarketing study in Taiwan.Medicine (Baltimore). 2019 Oct;98(42):e17171. doi: 10.1097/MD.0000000000017171. Medicine (Baltimore). 2019. PMID: 31626082 Free PMC article.
References
-
- French JA, Krauss GL, Steinhoff BJ, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54:117–125. - PubMed
-
- Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78:1408–1415. - PubMed
-
- Krauss GL, Perucca E, Ben-Menachem E, et al. Perampanel, a selective, noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist, as adjunctive therapy for refractory partial-onset seizures: interim results from phase III, extension study 307. Epilepsia. 2013;54:126–134. - PubMed
-
- Laurenza A, Ferry J, Hussein Z. Population pharmacokinetics and pharmacodynamics of perampanel: a pooled analysis from three phase III trials. Epilepsy Curr. 2012;12(Suppl. 1):2.231. Abstract.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

