Modeling the effects of β1-adrenergic receptor blockers and polymorphisms on cardiac myocyte Ca2+ handling
- PMID: 24867460
- PMCID: PMC4127930
- DOI: 10.1124/mol.113.090951
Modeling the effects of β1-adrenergic receptor blockers and polymorphisms on cardiac myocyte Ca2+ handling
Abstract
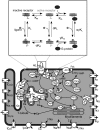
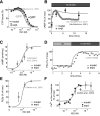
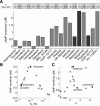
β-Adrenergic receptor blockers (β-blockers) are commonly used to treat heart failure, but the biologic mechanisms governing their efficacy are still poorly understood. The complexity of β-adrenergic signaling coupled with the influence of receptor polymorphisms makes it difficult to intuit the effect of β-blockers on cardiac physiology. While some studies indicate that β-blockers are efficacious by inhibiting β-adrenergic signaling, other studies suggest that they work by maintaining β-adrenergic responsiveness. Here, we use a systems pharmacology approach to test the hypothesis that in ventricular myocytes, these two apparently conflicting mechanisms for β-blocker efficacy can occur concurrently. We extended a computational model of the β(1)-adrenergic pathway and excitation-contraction coupling to include detailed receptor interactions for 19 ligands. Model predictions, validated with Ca(2+) and Förster resonance energy transfer imaging of adult rat ventricular myocytes, surprisingly suggest that β-blockers can both inhibit and maintain signaling depending on the magnitude of receptor stimulation. The balance of inhibition and maintenance of β(1)-adrenergic signaling is predicted to depend on the specific β-blocker (with greater responsiveness for metoprolol than carvedilol) and β(1)-adrenergic receptor Arg389Gly polymorphisms.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures




Similar articles
-
Characteristic effects of alpha1-beta1,2-adrenergic blocking agent, carvedilol, on [Ca2+]i in ventricular myocytes compared with those of timolol and atenolol.Circ J. 2003 Jan;67(1):83-90. doi: 10.1253/circj.67.83. Circ J. 2003. PMID: 12520158
-
Polydatin modulates Ca(2+) handling, excitation-contraction coupling and β-adrenergic signaling in rat ventricular myocytes.J Mol Cell Cardiol. 2012 Nov;53(5):646-56. doi: 10.1016/j.yjmcc.2012.08.009. Epub 2012 Aug 19. J Mol Cell Cardiol. 2012. PMID: 22921781
-
Sustained β-AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation.Life Sci. 2018 Jan 15;193:257-269. doi: 10.1016/j.lfs.2017.10.034. Epub 2017 Oct 28. Life Sci. 2018. PMID: 29107793
-
Positive inotropic stimulation.Curr Opin Crit Care. 2002 Oct;8(5):395-403. doi: 10.1097/00075198-200210000-00005. Curr Opin Crit Care. 2002. PMID: 12357106 Review.
-
Three Generations of β-blockers: History, Class Differences and Clinical Applicability.Curr Hypertens Rev. 2019;15(1):22-31. doi: 10.2174/1573402114666180918102735. Curr Hypertens Rev. 2019. PMID: 30227820 Review.
Cited by
-
Heart failure with reduced ejection fraction and atrial fibrillation: a Sub-Saharan African perspective.ESC Heart Fail. 2023 Jun;10(3):1580-1596. doi: 10.1002/ehf2.14332. Epub 2023 Mar 19. ESC Heart Fail. 2023. PMID: 36934444 Free PMC article. Review.
-
Network model-based screen for FDA-approved drugs affecting cardiac fibrosis.CPT Pharmacometrics Syst Pharmacol. 2021 Apr;10(4):377-388. doi: 10.1002/psp4.12599. Epub 2021 Feb 27. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33571402 Free PMC article.
-
Investigating β-adrenergic-induced cardiac hypertrophy through computational approach: classical and non-classical pathways.J Physiol Sci. 2018 Jul;68(4):503-520. doi: 10.1007/s12576-017-0557-5. Epub 2017 Jul 3. J Physiol Sci. 2018. PMID: 28674776 Free PMC article.
-
Molecular simulations reveal intricate coupling between agonist-bound β-adrenergic receptors and G protein.iScience. 2025 Jan 2;28(2):111741. doi: 10.1016/j.isci.2024.111741. eCollection 2025 Feb 21. iScience. 2025. PMID: 39898043 Free PMC article.
-
Molecular Pathophysiology of Congenital Long QT Syndrome.Physiol Rev. 2017 Jan;97(1):89-134. doi: 10.1152/physrev.00008.2016. Physiol Rev. 2017. PMID: 27807201 Free PMC article. Review.
References
-
- Allen MD, Zhang J. (2006) Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun 348:716–721 - PubMed
-
- Amanfu RK, Muller JB, and Saucerman JJ (2011). Automated image analysis of cardiac myocyte Ca2+ dynamics, in Proceedings of Engineering in Medicine and Biology Society, EMBC, 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Aug 30-Sept 3 2011, Boston, MA, pp. 4661–4664. - PMC - PubMed
-
- Bers DM, Lederer WJ, Berlin JR. (1990) Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am J Physiol 258:C944–C954 - PubMed
-
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. (1982) Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N Engl J Med 307:205–211 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

