Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2
- PMID: 24867643
- PMCID: PMC4032492
- DOI: 10.7554/eLife.02667
Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2
Abstract
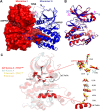
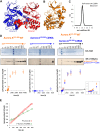
We elucidate the molecular mechanisms of two distinct activation strategies (autophosphorylation and TPX2-mediated activation) in human Aurora A kinase. Classic allosteric activation is in play where either activation loop phosphorylation or TPX2 binding to a conserved hydrophobic groove shifts the equilibrium far towards the active conformation. We resolve the controversy about the mechanism of autophosphorylation by demonstrating intermolecular autophosphorylation in a long-lived dimer by combining X-ray crystallography with functional assays. We then address the allosteric activation by TPX2 through activity assays and the crystal structure of a domain-swapped dimer of dephosphorylated Aurora A and TPX2(1-25). While autophosphorylation is the key regulatory mechanism in the centrosomes in the early stages of mitosis, allosteric activation by TPX2 of dephosphorylated Aurora A could be at play in the spindle microtubules. The mechanistic insights into autophosphorylation and allosteric activation by TPX2 binding proposed here, may have implications for understanding regulation of other protein kinases.DOI: http://dx.doi.org/10.7554/eLife.02667.001.
Keywords: activation; kinase; mechanism.
Copyright © 2014, Zorba et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

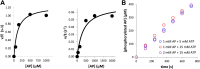
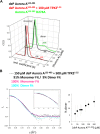
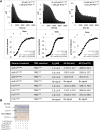
 , 0.01 ± 0.005 s−1) or T288V mutant Aurora A (
, 0.01 ± 0.005 s−1) or T288V mutant Aurora A ( , 0.05 ± 0.002 s−1) is increased by up to 50-fold (
, 0.05 ± 0.002 s−1) is increased by up to 50-fold ( , 0.5 ± 0.1 s−1) and 25-fold (
, 0.5 ± 0.1 s−1) and 25-fold ( , 1.2 ± 0.1 s−1), respectively, in the presence of TPX21−45. This rate is comparable to the kinetics of phosphorylated Aurora A in the absence of TPX21−45 (
, 1.2 ± 0.1 s−1), respectively, in the presence of TPX21−45. This rate is comparable to the kinetics of phosphorylated Aurora A in the absence of TPX21−45 ( , 1.0 ± 0.2 s−1). Phosphorylated Aurora A shows up to a twofold increase in AP kinetics in the presence of TPX21−45 (
, 1.0 ± 0.2 s−1). Phosphorylated Aurora A shows up to a twofold increase in AP kinetics in the presence of TPX21−45 ( , 2.3 ± 0.2 s−1). Reactions are carried out in the presence of 1 μM protein, 50 μM TPX21−45, 5 mM ATP, and 1 mM AP in assay buffer (20 mM TrisHCl, 200 mM NaCl, 20 mM MgCl2, 3% (vol/vol) glycerol, 1 mM TCEP, pH 7.50) at 25°C. Phosphorylated peptide production was monitored by reverse phase-high performance liquid chromatography (RP-HPLC). DOI:
, 2.3 ± 0.2 s−1). Reactions are carried out in the presence of 1 μM protein, 50 μM TPX21−45, 5 mM ATP, and 1 mM AP in assay buffer (20 mM TrisHCl, 200 mM NaCl, 20 mM MgCl2, 3% (vol/vol) glycerol, 1 mM TCEP, pH 7.50) at 25°C. Phosphorylated peptide production was monitored by reverse phase-high performance liquid chromatography (RP-HPLC). DOI:
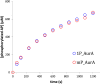
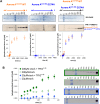
 , 0.0054 s−1) and 15 mM ATP (
, 0.0054 s−1) and 15 mM ATP ( , 0.0055 s−1) as well as 2 mM AP and 15 mM ATP (
, 0.0055 s−1) as well as 2 mM AP and 15 mM ATP ( , 0.0056 s−1). In each case, the kinetics of AP phosphorylation were identical within experimental error. Since the KM for ATP for WT Aurora A is about 10 μM (Kelly et al., 2011), all following kinetic reactions were run at 5 mM ATP (20 mM TrisHCl, 200 mM NaCl, 20 mM MgCl2, 3% [vol/vol] glycerol, 1 mM TCEP, pH 7.50) at 25°C. Phosphorylated peptide production was monitored by reverse phase-high performance liquid chromatography (RP-HPLC). DOI:
, 0.0056 s−1). In each case, the kinetics of AP phosphorylation were identical within experimental error. Since the KM for ATP for WT Aurora A is about 10 μM (Kelly et al., 2011), all following kinetic reactions were run at 5 mM ATP (20 mM TrisHCl, 200 mM NaCl, 20 mM MgCl2, 3% [vol/vol] glycerol, 1 mM TCEP, pH 7.50) at 25°C. Phosphorylated peptide production was monitored by reverse phase-high performance liquid chromatography (RP-HPLC). DOI:
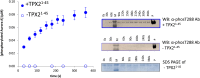

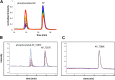






References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallographica Section D, Biological Crystallography 66:213–221. doi: 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Aliagas-Martin I, Burdick D, Corson L, Dotson J, Drummond J, Fields C, Huang OW, Hunsaker T, Kleinheinz T, Krueger E, Liang J, Moffat J, Phillips G, Pulk R, Rawson TE, Ultsch M, Walker L, Wiesmann C, Zhang B, Zhu BY, Cochran AG. 2009. A class of 2,4-bisanilinopyrimidine Aurora A inhibitors with unusually high selectivity against Aurora B. Journal of Medicinal Chemistry 52:3300–3307. doi: 10.1021/jm9000314 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

