Assessing the function of homologous recombination DNA repair in malignant pleural effusion (MPE) samples
- PMID: 24867690
- PMCID: PMC4090730
- DOI: 10.1038/bjc.2014.261
Assessing the function of homologous recombination DNA repair in malignant pleural effusion (MPE) samples
Abstract
Background: Patients with malignant pleural effusions (MPEs) generally have advanced disease with poor survival and few therapeutic options. Cells within MPEs may be used to stratify patients for targeted therapy. Targeted therapy with poly(ADP ribose) polymerase inhibitors (PARPi) depends on identifying homologous recombination DNA repair (HRR)-defective cancer cells. We aimed to determine the feasibility of assaying HRR status in MPE cells.
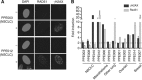
Methods: A total of 15 MPE samples were collected from consenting patients with non-small-cell lung cancer (NSCLC), mesothelioma and ovarian and breast cancer. Primary cultures were confirmed as epithelial by pancytokeratin, and HRR status was determined by the detection of γH2AX and RAD51 foci following a 24-h exposure to rucaparib, by immunofluorescence microscopy. Massively parallel next-generation sequencing of DNA repair genes was performed on cultured MPE cells.
Results: From 15 MPE samples, 13 cultures were successfully established, with HRR function successfully determined in 12 cultures. Four samples - three NSCLC and one mesothelioma - were HRR defective and eight samples - one NSCLC, one mesothelioma, one sarcomatoid, one breast and four ovarian cancers - were HRR functional. No mutations in DNA repair genes were associated with HRR status, but there was probable loss of heterozygosity of FANCG, RPA1 and PARP1.
Conclusions: HRR function can be successfully detected in MPE cells demonstrating the potential to stratify patients for targeted therapy with PARPi.
Figures


Similar articles
-
Detection of impaired homologous recombination repair in NSCLC cells and tissues.J Thorac Oncol. 2013 Mar;8(3):279-86. doi: 10.1097/JTO.0b013e31827ecf83. J Thorac Oncol. 2013. PMID: 23399959 Free PMC article.
-
Detecting EGFR mutations and ALK/ROS1 rearrangements in non-small cell lung cancer using malignant pleural effusion samples.Thorac Cancer. 2019 Feb;10(2):193-202. doi: 10.1111/1759-7714.12932. Epub 2018 Dec 19. Thorac Cancer. 2019. PMID: 30565433 Free PMC article.
-
The diagnostic accuracy of pleural effusion and plasma samples versus tumour tissue for detection of EGFR mutation in patients with advanced non-small cell lung cancer: comparison of methodologies.J Clin Pathol. 2013 Dec;66(12):1065-9. doi: 10.1136/jclinpath-2013-201728. Epub 2013 Jul 25. J Clin Pathol. 2013. PMID: 23888061 Free PMC article.
-
Novel insights into the systemic treatment of lung cancer malignant pleural effusion.Clin Respir J. 2019 Mar;13(3):131-138. doi: 10.1111/crj.13005. Clin Respir J. 2019. PMID: 30737898 Review.
-
Repositioning PARP inhibitors in the treatment of thoracic malignancies.Cancer Treat Rev. 2021 Sep;99:102256. doi: 10.1016/j.ctrv.2021.102256. Epub 2021 Jul 7. Cancer Treat Rev. 2021. PMID: 34261032 Review.
Cited by
-
BRCAness revisited.Nat Rev Cancer. 2016 Feb;16(2):110-20. doi: 10.1038/nrc.2015.21. Epub 2016 Jan 18. Nat Rev Cancer. 2016. PMID: 26775620 Review.
-
Sensitizing Ewing sarcoma to chemo- and radiotherapy by inhibition of the DNA-repair enzymes DNA protein kinase (DNA-PK) and poly-ADP-ribose polymerase (PARP) 1/2.Oncotarget. 2017 Sep 28;8(69):113418-113430. doi: 10.18632/oncotarget.21300. eCollection 2017 Dec 26. Oncotarget. 2017. PMID: 29371919 Free PMC article.
-
PARP-1: a critical regulator in radioprotection and radiotherapy-mechanisms, challenges, and therapeutic opportunities.Front Pharmacol. 2023 Jun 6;14:1198948. doi: 10.3389/fphar.2023.1198948. eCollection 2023. Front Pharmacol. 2023. PMID: 37351512 Free PMC article. Review.
-
The RAD51-FFPE Test; Calibration of a Functional Homologous Recombination Deficiency Test on Diagnostic Endometrial and Ovarian Tumor Blocks.Cancers (Basel). 2021 Jun 15;13(12):2994. doi: 10.3390/cancers13122994. Cancers (Basel). 2021. PMID: 34203855 Free PMC article.
-
Poly(ADP-ribose) polymerase inhibition: past, present and future.Nat Rev Drug Discov. 2020 Oct;19(10):711-736. doi: 10.1038/s41573-020-0076-6. Epub 2020 Sep 3. Nat Rev Drug Discov. 2020. PMID: 32884152 Review.
References
-
- Birkelbach M, Ferraiolo N, Gheorghiu L, Pfaffle HN, Daly B, Ebright MI, Spencer C, O'Hara C, Whetstine JR, Benes CH, Sequist LV, Zou L, Dahm-Daphi J, Kachnic LA, Willers H. Detection of impaired homologous recombination repair in NSCLC cells and tissues. J Thorac Oncol. 2013;8 (3):279–286. - PMC - PubMed
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434 (7035):913–917. - PubMed
-
- CRUK 2013Cancer Statistics Report: Cancer Mortality in the UK in 2010.
-
- Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12 (12):801–817. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

