Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation
- PMID: 24867695
- PMCID: PMC4090735
- DOI: 10.1038/bjc.2014.272
Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation
Abstract
Background: Targeting both mitochondrial bioenergetics and glycolysis pathway is an effective way to inhibit proliferation of tumour cells, including those that are resistant to conventional chemotherapeutics.
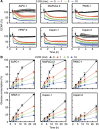
Methods: In this study, using the Seahorse 96-well Extracellular Flux Analyzer, we mapped the two intrinsic cellular bioenergetic parameters, oxygen consumption rate and proton production rate in six different pancreatic cancer cell lines and determined their differential sensitivity to mitochondrial and glycolytic inhibitors.
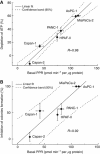
Results: There exists a very close relationship among intracellular bioenergetic parameters, depletion of ATP and anti-proliferative effects (inhibition of colony-forming ability) in pancreatic cancer cells derived from different genetic backgrounds treated with the glycolytic inhibitor, 2-deoxyglucose (2-DG). The most glycolytic pancreatic cancer cell line was exquisitely sensitive to 2-DG, whereas the least glycolytic pancreatic cancer cell was resistant to 2-DG. However, when combined with metformin, inhibitor of mitochondrial respiration and activator of AMP-activated protein kinase, 2-DG synergistically enhanced ATP depletion and inhibited cell proliferation even in poorly glycolytic, 2-DG-resistant pancreatic cancer cell line. Furthermore, treatment with conventional chemotherapeutic drugs (e.g., gemcitabine and doxorubicin) or COX-2 inhibitor, celecoxib, sensitised the cells to 2-DG treatment.
Conclusions: Detailed profiling of cellular bioenergetics can provide new insight into the design of therapeutic strategies for inhibiting pancreatic cancer cell metabolism and proliferation.
Figures



References
-
- American Cancer Society . Cancer Facts & Figures 2013. American Cancer Society: Atlanta, GA, USA; 2013. pp. 25–34.
-
- Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. - PubMed
-
- Bernal SD, Lampidis TJ, McIsaac RM, Chen LB. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983;222:169–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

