Natrelle round silicone breast implants: Core Study results at 10 years
- PMID: 24867717
- PMCID: PMC4819531
- DOI: 10.1097/PRS.0000000000000021
Natrelle round silicone breast implants: Core Study results at 10 years
Abstract
Background: Allergan's Natrelle round silicone-filled breast implants were approved by the U.S. Food and Drug Administration in 2006 based on interim results from the Core Study; final 10-year study results are now available.
Methods: Seven hundred fifteen subjects were implanted with smooth and Biocell textured Natrelle round silicone implants and attended clinic visits at 0 to 4 weeks, 6 months, 1 year, and annually through 10 years. Approximately one-third of subjects underwent magnetic resonance imaging at years 1, 3, 5, 7, and 9 to assess rupture.
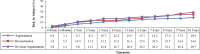
Results: Complication rates showed modest increases over the previously published 6-year rates. The Kaplan-Meier capsular contracture rate was 18.9 percent for augmentation, 28.7 percent for revision-augmentation, and 24.6 percent for reconstruction. Among augmentation subjects, capsular contracture was significantly lower (p = 0.023) for submuscular (15.7 percent) versus subglandular (26.3 percent) placement. The overall rupture rate in the magnetic resonance imaging cohort was 13.0 percent for subjects and 7.7 percent for implants. By the end of the study, 81.8 percent of augmentation subjects still had an original implant in place. Using a five-point scale, 94.2 percent of augmentation, 83.8 percent of revision-augmentation, and 90.7 percent of reconstruction subjects reported being satisfied or definitely satisfied with their implants. Significant improvement over baseline was also seen in overall breast satisfaction and satisfaction with breast size, shape, feel, and how well they matched.
Conclusion: The 10-year data from the Natrelle Core Study, which can guide surgeons and patients in decision-making, demonstrate safety and high levels of patient satisfaction.
Clinical question/level of evidence: Therapeutic, III.
Trial registration: ClinicalTrials.gov NCT00689871.
Conflict of interest statement
Figures
Comment in
-
Discussion: Natrelle round silicone breast implants: core study results at 10 years.Plast Reconstr Surg. 2014 Jun;133(6):1362-1363. doi: 10.1097/PRS.0000000000000167. Plast Reconstr Surg. 2014. PMID: 24867718 No abstract available.
References
-
- Jewell ML. Silicone gel breast implants at 50: The state of the science. Aesthet Surg J. 2012;32:1031–1034. - PubMed
-
- Spear SL, Murphy DK, Slicton A, Walker PS Inamed Silicone Breast Implant U.S. Study Group. Inamed silicone breast implant core study results at 6 years. Plast Reconstr Surg. 2007;120(Suppl 1):8S–16S; discussion 17S. - PubMed
-
- Murphy DK, Beckstrand M, Sarwer DB. A prospective, multi-center study of psychosocial outcomes after augmentation with natrelle silicone-filled breast implants. Ann Plast Surg. 2009;62:118–121. - PubMed
-
- Gladfelter J, Murphy D. Breast augmentation motivations and satisfaction: A prospective study of more than 3,000 silicone implantations. Plast Surg Nurs. 2008;28:170–174; quiz 175. - PubMed
-
- Com-Nougue C, Rodary C, Patte C. How to establish equivalence when data are censored: A randomized trial of treatments for B non-Hodgkin lymphoma. Stat Med. 1993;12:1353–1364. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials