OPT3 Is a Phloem-Specific Iron Transporter That Is Essential for Systemic Iron Signaling and Redistribution of Iron and Cadmium in Arabidopsis
- PMID: 24867923
- PMCID: PMC4079381
- DOI: 10.1105/tpc.114.123737
OPT3 Is a Phloem-Specific Iron Transporter That Is Essential for Systemic Iron Signaling and Redistribution of Iron and Cadmium in Arabidopsis
Abstract
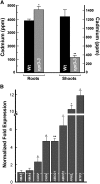
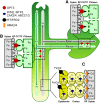
Iron is essential for both plant growth and human health and nutrition. Knowledge of the signaling mechanisms that communicate iron demand from shoots to roots to regulate iron uptake as well as the transport systems mediating iron partitioning into edible plant tissues is critical for the development of crop biofortification strategies. Here, we report that OPT3, previously classified as an oligopeptide transporter, is a plasma membrane transporter capable of transporting transition ions in vitro. Studies in Arabidopsis thaliana show that OPT3 loads iron into the phloem, facilitates iron recirculation from the xylem to the phloem, and regulates both shoot-to-root iron signaling and iron redistribution from mature to developing tissues. We also uncovered an aspect of crosstalk between iron homeostasis and cadmium partitioning that is mediated by OPT3. Together, these discoveries provide promising avenues for targeted strategies directed at increasing iron while decreasing cadmium density in the edible portions of crops and improving agricultural productivity in iron deficient soils.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures
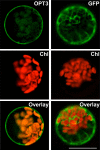
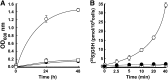

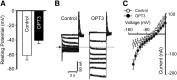

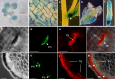


Similar articles
-
OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds.Mol Plant. 2014 Sep;7(9):1455-1469. doi: 10.1093/mp/ssu067. Epub 2014 May 31. Mol Plant. 2014. PMID: 24880337 Free PMC article.
-
Changes in iron availability in Arabidopsis are rapidly sensed in the leaf vasculature and impaired sensing leads to opposite transcriptional programs in leaves and roots.Plant Cell Environ. 2018 Oct;41(10):2263-2276. doi: 10.1111/pce.13192. Epub 2018 Jun 19. Plant Cell Environ. 2018. PMID: 29520929
-
Loss of OPT3 function decreases phloem copper levels and impairs crosstalk between copper and iron homeostasis and shoot-to-root signaling in Arabidopsis thaliana.Plant Cell. 2023 May 29;35(6):2157-2185. doi: 10.1093/plcell/koad053. Plant Cell. 2023. PMID: 36814393 Free PMC article.
-
Local and systemic signaling of iron status and its interactions with homeostasis of other essential elements.Front Plant Sci. 2015 Sep 14;6:716. doi: 10.3389/fpls.2015.00716. eCollection 2015. Front Plant Sci. 2015. PMID: 26442030 Free PMC article. Review.
-
The potassium battery: a mobile energy source for transport processes in plant vascular tissues.New Phytol. 2017 Dec;216(4):1049-1053. doi: 10.1111/nph.14667. Epub 2017 Jun 23. New Phytol. 2017. PMID: 28643868 Review.
Cited by
-
shrunken4 is a mutant allele of ZmYSL2 that affects aleurone development and starch synthesis in maize.Genetics. 2021 Jun 24;218(2):iyab070. doi: 10.1093/genetics/iyab070. Genetics. 2021. PMID: 34009311 Free PMC article.
-
PRC2-mediated H3K27me3 modulates shoot iron homeostasis in Arabidopsis thaliana.Plant Signal Behav. 2020 Sep 1;15(9):1784549. doi: 10.1080/15592324.2020.1784549. Epub 2020 Jun 27. Plant Signal Behav. 2020. PMID: 32594838 Free PMC article.
-
Genome-wide analysis indicates diverse physiological roles of the turnip (Brassica rapa var. rapa) oligopeptide transporters gene family.Plant Divers. 2018 Mar 12;40(2):57-67. doi: 10.1016/j.pld.2018.03.001. eCollection 2018 Apr. Plant Divers. 2018. PMID: 30159543 Free PMC article.
-
The Pseudomonas fluorescens Siderophore Pyoverdine Weakens Arabidopsis thaliana Defense in Favor of Growth in Iron-Deficient Conditions.Plant Physiol. 2016 May;171(1):675-93. doi: 10.1104/pp.15.01537. Epub 2016 Mar 8. Plant Physiol. 2016. PMID: 26956666 Free PMC article.
-
Iron homeostasis in plants - a brief overview.Metallomics. 2017 Jul 19;9(7):813-823. doi: 10.1039/c7mt00136c. Metallomics. 2017. PMID: 28686269 Free PMC article. Review.
References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Amasheh S., Weber W. (1999). Further characteristics of the Ca(2+)-inactivated Cl(-) channel in Xenopus laevis oocytes. J. Membr. Biol. 172: 169–179 - PubMed
-
- Arrivault S., Senger T., Krämer U. (2006). The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. Plant J. 46: 861–879 - PubMed
-
- Arteca R.N., Arteca J.M. (2000). A novel method for growing Arabidopsis thaliana plants hydroponically. Physiol. Plant. 108: 188–193
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

