Activated hepatic stellate cells are dependent on self-collagen, cleaved by membrane type 1 matrix metalloproteinase for their growth
- PMID: 24867951
- PMCID: PMC4106337
- DOI: 10.1074/jbc.M113.544494
Activated hepatic stellate cells are dependent on self-collagen, cleaved by membrane type 1 matrix metalloproteinase for their growth
Abstract
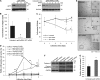
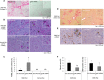
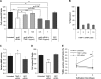
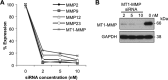
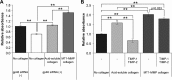
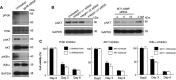
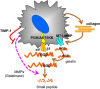
Stellate cells are distributed throughout organs, where, upon chronic damage, they become activated and proliferate to secrete collagen, which results in organ fibrosis. An intriguing property of hepatic stellate cells (HSCs) is that they undergo apoptosis when collagen is resolved by stopping tissue damage or by treatment, even though the mechanisms are unknown. Here we disclose the fact that HSCs, normal diploid cells, acquired dependence on collagen for their growth during the transition from quiescent to active states. The intramolecular RGD motifs of collagen were exposed by cleavage with their own membrane type 1 matrix metalloproteinase (MT1-MMP). The following evidence supports this conclusion. When rat activated HSCs (aHSCs) were transduced with siRNA against the collagen-specific chaperone gp46 to inhibit collagen secretion, the cells underwent autophagy followed by apoptosis. Concomitantly, the growth of aHSCs was suppressed, whereas that of quiescent HSCs was not. These in vitro results are compatible with the in vivo observation that apoptosis of aHSCs was induced in cirrhotic livers of rats treated with siRNAgp46. siRNA against MT1-MMP and addition of tissue inhibitor of metalloproteinase 2 (TIMP-2), which mainly inhibits MT1-MMP, also significantly suppressed the growth of aHSCs in vitro. The RGD inhibitors echistatin and GRGDS peptide and siRNA against the RGD receptor αVβ1 resulted in the inhibition of aHSCs growth. Transduction of siRNAs against gp46, αVβ1, and MT1-MMP to aHSCs inhibited the survival signal of PI3K/AKT/IκB. These results could provide novel antifibrosis strategies.
Keywords: Apoptosis; Extracellular Matrix; Fibrosis; Heat Shock Protein (HSP); Integrin.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Zhao L., Burt A. D. (2007) The diffuse stellate cell system. J. Mol. Histol. 38, 53–64 - PubMed
-
- Boulter L., Govaere O., Bird T. G., Radulescu S., Ramachandran P., Pellicoro A., Ridgway R. A., Seo S. S., Spee B., Van Rooijen N., Sansom O. J., Iredale J. P., Lowell S., Roskams T., Forbes S. J. (2012) Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 - PMC - PubMed
-
- Hemmann S., Graf J., Roderfeld M., Roeb E. (2007) Expression of MMPs and TIMPs in liver fibrosis: a systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 46, 955–975 - PubMed
-
- Iredale J. P., Benyon R. C., Pickering J., McCullen M., Northrop M., Pawley S., Hovell C., Arthur M. J. (1998) Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

