Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer
- PMID: 24868022
- PMCID: PMC4164758
- DOI: 10.1200/JCO.2013.49.3676
Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer
Abstract
Purpose: To compare the risk of hospitalization between patients with early-stage breast cancer who received different chemotherapy regimens.
Patient and methods: We identified 3,567 patients older than age 65 years from the SEER/Texas Cancer Registry-Medicare database and 9,327 patients younger than age 65 years from the MarketScan database who were diagnosed with early-stage breast cancer between 2003 and 2007. The selection was nonrandomized and nonprospectively collected. We categorized patients according to the regimens they received: docetaxel (T) and cyclophosphamide (C), doxorubicin (A) and C, TAC, AC + T, dose-dense AC + paclitaxel (P) or AC + weekly P. We compared the rates of chemotherapy-related hospitalizations that occurred within 6 months of chemotherapy initiation and used multivariable logistic regression analysis to identify the factors associated with these hospitalizations.
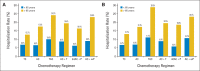
Results: Among patients younger than age 65 years, the hospitalization rates ranged from 6.2% (dose-dense AC + P) to 10.0% (TAC), and those who received TAC and AC + T had significantly higher rates of hospitalization than did patients who received TC. Among patients older than age 65 years, these rates ranged from 12.7% (TC) to 24.2% (TAC) and the rates of hospitalization of patients who received TAC, AC + T, AC, or AC + weekly P were higher than those of patients who received TC.
Conclusion: TAC and AC + T were associated with the highest risk of hospitalization in patients younger than age 65 years. Among patients older than age 65 years, all regimens (aside from dose-dense AC + P) were associated with a higher risk of hospitalization than TC. Results may be affected by selection biases where less aggressive regimens are offered to frailer patients.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Enhancing therapeutic decision making when options abound: toxicities matter.J Clin Oncol. 2014 Jul 1;32(19):1990-3. doi: 10.1200/JCO.2014.55.1903. Epub 2014 May 27. J Clin Oncol. 2014. PMID: 24868027 No abstract available.
References
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. - PubMed
-
- Pal SK, Childs BH, Pegram M. Emergence of nonanthracycline regimens in the adjuvant treatment of breast cancer. Breast Cancer Res Treat. 2010;119:25–32. - PubMed
-
- Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. - PubMed
-
- Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

