U.S. Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations
- PMID: 24868098
- PMCID: PMC4077454
- DOI: 10.1634/theoncologist.2014-0089
U.S. Food and Drug Administration approval summary: Erlotinib for the first-line treatment of metastatic non-small cell lung cancer with epidermal growth factor receptor exon 19 deletions or exon 21 (L858R) substitution mutations
Abstract
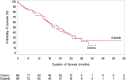
On May 14, 2013, the U.S. Food and Drug Administration approved erlotinib (Tarceva, Astellas Pharma Inc., Northbrook, IL, http://www.us.astellas.com/) for the first-line treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations. This indication for erlotinib was approved concurrently with the cobas EGFR Mutation Test (Roche Molecular Systems, Inc., Basel, Switzerland, http://www.molecular.roche.com), a companion diagnostic test for patient selection. The approval was based on clinically important improvements in progression-free survival (PFS) and objective response rate (ORR) and an acceptable toxicity profile demonstrated in a multicenter, open label trial enrolling 174 patients with metastatic NSCLC whose tumors had EGFR mutations as determined by a laboratory-developed test. Patients were randomized (1:1) to receive erlotinib (150 mg/day) or platinum-based doublet chemotherapy. The primary endpoint was investigator-assessed PFS. Secondary endpoints included overall survival (OS) and ORR. Superior PFS (hazard ratio [HR] 0.34; 95% confidence interval [CI]: 0.23, 0.49; p < .001) and ORR (65% vs. 16%) were observed in the erlotinib arm. Median PFS was 10.4 months and 5.2 months in the erlotinib and chemotherapy arms, respectively. There was no difference in OS (HR 0.93; 95% CI: 0.64, 1.35) with median OS of 22.9 months and 19.5 months in the erlotinib and chemotherapy arms, respectively. The most frequent (≥30%) adverse reactions in the erlotinib-treated patients were rash, diarrhea, asthenia, cough, dyspnea, and decreased appetite. The most frequent (≥5%) grade 3 and 4 adverse reactions were rash and diarrhea.
[仅摘要] 摘要
2013 年 5 月 14 日,美国食品药品监督管理局批准将厄洛替尼(特罗凯,安斯泰来制药公司,伊利诺伊州诺斯布鲁克市,
Keywords: EGFR mutations; Erlotinib; FDA; Lung cancer.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Marino P, Pampallona S, Preatoni A, et al. Chemotherapy vs. supportive care in advanced non-small-cell lung cancer: Results of a meta-analysis of the literature. Chest. 1994;106:861–865. - PubMed
-
- Kelly K, Crowley J, Bunn PA, Jr., et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: A Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. - PubMed
-
- Scagliotti GV, De Marinis F, Rinaldi M, et al. Italian Lung Cancer Project Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. - PubMed
-
- Fossella F, Pereira JR, von Pawel J, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: The TAX 326 study group. J Clin Oncol. 2003;21:3016–3024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous