Salidroside stimulates mitochondrial biogenesis and protects against H₂O₂-induced endothelial dysfunction
- PMID: 24868319
- PMCID: PMC4020198
- DOI: 10.1155/2014/904834
Salidroside stimulates mitochondrial biogenesis and protects against H₂O₂-induced endothelial dysfunction
Abstract
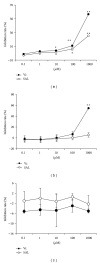
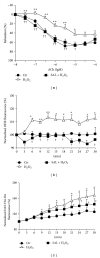
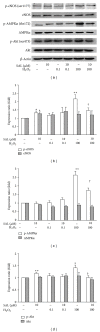
Salidroside (SAL) is an active component of Rhodiola rosea with documented antioxidative properties. The purpose of this study is to explore the mechanism of the protective effect of SAL on hydrogen peroxide- (H2O2-) induced endothelial dysfunction. Pretreatment of the human umbilical vein endothelial cells (HUVECs) with SAL significantly reduced the cytotoxicity brought by H2O2. Functional studies on the rat aortas found that SAL rescued the endothelium-dependent relaxation and reduced superoxide anion (O2(∙-)) production induced by H2O2. Meanwhile, SAL pretreatment inhibited H2O2-induced nitric oxide (NO) production. The underlying mechanisms involve the inhibition of H2O2-induced activation of endothelial nitric oxide synthase (eNOS), adenosine monophosphate-activated protein kinase (AMPK), and Akt, as well as the redox sensitive transcription factor, NF-kappa B (NF- κ B). SAL also increased mitochondrial mass and upregulated the mitochondrial biogenesis factors, peroxisome proliferator-activated receptor gamma-coactivator-1alpha (PGC-1 α ), and mitochondrial transcription factor A (TFAM) in the endothelial cells. H2O2-induced mitochondrial dysfunction, as demonstrated by reduced mitochondrial membrane potential (Δ ψ m) and ATP production, was rescued by SAL pretreatment. Taken together, these findings implicate that SAL could protect endothelium against H2O2-induced injury via promoting mitochondrial biogenesis and function, thus preventing the overactivation of oxidative stress-related downstream signaling pathways.
Figures




Similar articles
-
Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway.Vascul Pharmacol. 2015 Sep;72:141-52. doi: 10.1016/j.vph.2015.07.004. Epub 2015 Jul 15. Vascul Pharmacol. 2015. PMID: 26187353
-
Salidroside protects against homocysteine-induced injury in human umbilical vein endothelial cells via the regulation of endoplasmic reticulum stress.Cardiovasc Ther. 2017 Feb;35(1):33-39. doi: 10.1111/1755-5922.12234. Cardiovasc Ther. 2017. PMID: 27809414
-
Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation.Mol Med Rep. 2013 Jul;8(1):147-53. doi: 10.3892/mmr.2013.1468. Epub 2013 May 9. Mol Med Rep. 2013. PMID: 23660824
-
Pharmacological effects of salidroside on central nervous system diseases.Biomed Pharmacother. 2022 Dec;156:113746. doi: 10.1016/j.biopha.2022.113746. Epub 2022 Oct 10. Biomed Pharmacother. 2022. PMID: 36228376 Review.
-
Mechanisms of cerebrovascular protection: oestrogen, inflammation and mitochondria.Acta Physiol (Oxf). 2011 Sep;203(1):149-54. doi: 10.1111/j.1748-1716.2010.02184.x. Epub 2010 Oct 11. Acta Physiol (Oxf). 2011. PMID: 20825371 Free PMC article. Review.
Cited by
-
Effects of rhodiola crenulata on mice hearts under severe sleep apnea.BMC Complement Altern Med. 2015 Jun 25;15:198. doi: 10.1186/s12906-015-0698-0. BMC Complement Altern Med. 2015. PMID: 26108210 Free PMC article.
-
Salidroside mitigates hypoxia/reoxygenation injury by alleviating endoplasmic reticulum stress‑induced apoptosis in H9c2 cardiomyocytes.Mol Med Rep. 2018 Oct;18(4):3760-3768. doi: 10.3892/mmr.2018.9403. Epub 2018 Aug 20. Mol Med Rep. 2018. Retraction in: Mol Med Rep. 2024 Nov;30(5):196. doi: 10.3892/mmr.2024.13320. PMID: 30132527 Free PMC article. Retracted.
-
CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis.Autophagy. 2020 Jun;16(6):1111-1129. doi: 10.1080/15548627.2019.1659613. Epub 2019 Sep 4. Autophagy. 2020. PMID: 31448673 Free PMC article.
-
Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction.Oxid Med Cell Longev. 2018 Apr 18;2018:9086947. doi: 10.1155/2018/9086947. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29849923 Free PMC article.
-
Antioxidant Effects of Salidroside in the Cardiovascular System.Evid Based Complement Alternat Med. 2020 Sep 26;2020:9568647. doi: 10.1155/2020/9568647. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33062029 Free PMC article. Review.
References
-
- Hermida N, Balligand JL. Low-density lipoprotein-cholesterol-induced endothelial dysfunction and oxidative stress: the role of statins. Antioxidants & Redox Signaling. 2013;20(8):1216–1237. - PubMed
-
- Sena CM, Pereira AM, Seica R. Endothelial dysfunction—a major mediator of diabetic vascular disease. Biochimica et Biophysica Acta. 2013;1832(12):2216–2231. - PubMed
-
- Moens AL, Kietadisorn R, Lin JY, Kass D. Targeting endothelial and myocardial dysfunction with tetrahydrobiopterin. Journal of Molecular and Cellular Cardiology. 2011;51(4):559–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

