Design and execution of clinical trials in orthopaedic surgery
- PMID: 24869465
- PMCID: PMC4097861
- DOI: 10.1302/2046-3758.35.2000280
Design and execution of clinical trials in orthopaedic surgery
Abstract
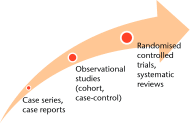
High-quality randomised controlled trials (RCTs) evaluating surgical therapies are fundamental to the delivery of evidence-based orthopaedics. Orthopaedic clinical trials have unique challenges; however, when these challenges are overcome, evidence from trials can be definitive in its impact on surgical practice. In this review, we highlight several issues that pose potential challenges to orthopaedic investigators aiming to perform surgical randomised controlled trials. We begin with a discussion on trial design issues, including the ethics of sham surgery, the importance of sample size, the need for patient-important outcomes, and overcoming expertise bias. We then explore features surrounding the execution of surgical randomised trials, including ethics review boards, the importance of organisational frameworks, and obtaining adequate funding. Cite this article: Bone Joint Res 2014;3:161-8.
Keywords: Orthopaedics; Randomised Controlled Trials; Study Design.
©2014 The British Editorial Society of Bone & Joint Surgery.
Conflict of interest statement
Figures
References
-
- Campbell AJ, Bagley A, Van Heest A, James MA. Challenges of randomized controlled surgical trials. Orthop Clin North Am 2010;41:145–155 - PubMed
-
- Bhandari M, Richards RR, Sprague S, Schemitsch EH. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. J Bone Joint Surg [Am] 2002;84-A:388–396 - PubMed
-
- Poolman RW, Struijs PA, Krips R, et al. Reporting of outcomes in orthopaedic randomized trials: does blinding of outcome assessors matter? J Bone Joint Surg [Am] 2007;89-A:550–558 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources