Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia
- PMID: 24871132
- PMCID: PMC4501573
- DOI: 10.1126/scitranslmed.3008004
Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia
Abstract
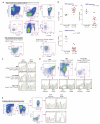
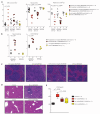
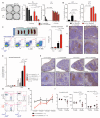
Hairy cell leukemia (HCL) is a chronic lymphoproliferative disorder characterized by somatic BRAFV600E mutations. The malignant cell in HCL has immunophenotypic features of a mature B cell, but no normal counterpart along the continuum of developing B lymphocytes has been delineated as the cell of origin. We find that the BRAFV600E mutation is present in hematopoietic stem cells (HSCs) in HCL patients, and that these patients exhibit marked alterations in hematopoietic stem/progenitor cell (HSPC) frequencies. Quantitative sequencing analysis revealed a mean BRAFV600E-mutant allele frequency of 4.97% in HSCs from HCL patients. Moreover, transplantation of BRAFV600E-mutant HSCs from an HCL patient into immunodeficient mice resulted in stable engraftment of BRAFV600E-mutant human hematopoietic cells, revealing the functional self-renewal capacity of HCL HSCs. Consistent with the human genetic data, expression of BRafV600E in murine HSPCs resulted in a lethal hematopoietic disorder characterized by splenomegaly, anemia, thrombocytopenia, increased circulating soluble CD25, and increased clonogenic capacity of B lineage cells-all classic features of human HCL. In contrast, restricting expression of BRafV600E to the mature B cell compartment did not result in disease. Treatment of HCL patients with vemurafenib, an inhibitor of mutated BRAF, resulted in normalization of HSPC frequencies and increased myeloid and erythroid output from HSPCs. These findings link the pathogenesis of HCL to somatic mutations that arise in HSPCs and further suggest that chronic lymphoid malignancies may be initiated by aberrant HSCs.
Copyright © 2014, American Association for the Advancement of Science.
Figures


References
-
- Golomb H, Davis S, Wilson C, Vardiman J. Surface immunoglobulins on hairy cells of 55 patients with hairy cell leukemia. Am. J. Hematol. 1982;12:397–401. - PubMed
-
- Forconi F, Sahota S, Raspadori D, Mockridge C, Lauria F, Stevenson F. Tumor cells of hairy cell leukemia express multiple clonally related immunoglobulin isotypes via RNA splicing. Blood. 2001;98:1174–1181. - PubMed
-
- Maloum K, Magnac C, Azgui Z, Cau C, Charlotte F, Binet J, Merle-Béral H, Dighiero G. VH gene expression in hairy cell leukaemia. Br. J. Haematol. 1998;101:171–178. - PubMed
-
- Polliack A. Hairy cell leukemia: Biology, clinical diagnosis, unusual manifestations and associated disorders. Rev. Clin. Exp. Hematol. 2002;6:366–388. - PubMed
-
- Tiacci E, Liso A, Piris M, Falini B. Evolving concepts in the pathogenesis of hairy-cell leukaemia. Nat. Rev. Cancer. 2006;6:437–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

