Field study of dried blood spot specimens for HIV-1 drug resistance genotyping
- PMID: 24871219
- PMCID: PMC4136191
- DOI: 10.1128/JCM.00544-14
Field study of dried blood spot specimens for HIV-1 drug resistance genotyping
Abstract
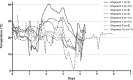
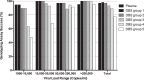
Dried blood spots (DBS) are an alternative specimen type for HIV drug resistance genotyping in resource-limited settings. Data relating to the impact of DBS storage and shipment conditions on genotyping efficiency under field conditions are limited. We compared the genotyping efficiencies and resistance profiles of DBS stored and shipped at different temperatures to those of plasma specimens collected in parallel from patients receiving antiretroviral therapy in Uganda. Plasma and four DBS cards from anti-coagulated venous blood and a fifth card from finger-prick blood were prepared from 103 HIV patients with a median viral load (VL) of 57,062 copies/ml (range, 1,081 to 2,964,191). DBS were stored at ambient temperature for 2 or 4 weeks or frozen at -80 °C and shipped from Uganda to the United States at ambient temperature or frozen on dry ice for genotyping using a broadly sensitive in-house method. Plasma (97.1%) and DBS (98.1%) stored and shipped frozen had similar genotyping efficiencies. DBS stored frozen (97.1%) or at ambient temperature for 2 weeks (93.2%) and shipped at ambient temperature also had similar genotyping efficiencies. Genotyping efficiency was reduced for DBS stored at ambient temperature for 4 weeks (89.3%, P = 0.03) or prepared from finger-prick blood and stored at ambient temperature for 2 weeks (77.7%, P < 0.001) compared to DBS prepared from venous blood and handled similarly. Resistance profiles were similar between plasma and DBS specimens. This report delineates the optimal DBS collection, storage, and shipping conditions and opens a new avenue for cost-saving ambient-temperature DBS specimen shipments for HIV drug resistance (HIVDR) surveillances in resource-limited settings.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- World Health Organization. 2013. Global update on HIV treatment 2013: results, impact and opportunities. World Health Organization. http://www.who.int/hiv/pub/progressreports/update2013/en/index.html Accessed 24 September 2013
-
- Frentz D, Boucher CA, van de Vijver DA. 2012. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 14:17–27 http://www.aidsreviews.com/files/2012_14_1_017-027.pdf - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

