A novel application of the Intent to Attend assessment to reduce bias due to missing data in a randomized controlled clinical trial
- PMID: 24872362
- PMCID: PMC4247354
- DOI: 10.1177/1740774514531096
A novel application of the Intent to Attend assessment to reduce bias due to missing data in a randomized controlled clinical trial
Abstract
Background: Missing data are unavoidable in most randomized controlled clinical trials, especially when measurements are taken repeatedly. If strong assumptions about the missing data are not accurate, crude statistical analyses are biased and can lead to false inferences. Furthermore, if we fail to measure all predictors of missing data, we may not be able to model the missing data process sufficiently. In longitudinal randomized trials, measuring a patient's intent to attend future study visits may help to address both of these problems. Leon et al. developed and included the Intent to Attend assessment in the Lithium Treatment - Moderate dose Use Study (LiTMUS), aiming to remove bias due to missing data from the primary study hypothesis.
Purpose: The purpose of this study is to assess the performance of the Intent to Attend assessment with regard to its use in a sensitivity analysis of missing data.
Methods: We fit marginal models to assess whether a patient's self-rated intent predicted actual study adherence. We applied inverse probability of attrition weighting (IPAW) coupled with patient intent to assess whether there existed treatment group differences in response over time. We compared the IPAW results to those obtained using other methods.
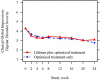
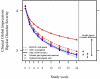
Results: Patient-rated intent predicted missed study visits, even when adjusting for other predictors of missing data. On average, the hazard of retention increased by 19% for every one-point increase in intent. We also found that more severe mania, male gender, and a previously missed visit predicted subsequent absence. Although we found no difference in response between the randomized treatment groups, IPAW increased the estimated group difference over time.
Limitations: LiTMUS was designed to limit missed study visits, which may have attenuated the effects of adjusting for missing data. Additionally, IPAW can be less efficient and less powerful than maximum likelihood or Bayesian estimators, given that the parametric model is well specified.
Conclusions: In LiTMUS, the Intent to Attend assessment predicted missed study visits. This item was incorporated into our IPAW models and helped reduce bias due to informative missing data. This analysis should both encourage and facilitate future use of the Intent to Attend assessment along with IPAW to address missing data in a randomized trial.
© The Author(s), 2014.
Figures


Similar articles
-
To attend, or not to attend: Examining caregiver intentions and study compliance in a pediatric, randomized controlled trial.Clin Trials. 2020 Apr;17(2):223-230. doi: 10.1177/1740774519893307. Epub 2020 Jan 27. Clin Trials. 2020. PMID: 31984781 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Bias reduction with an adjustment for participants' intent to dropout of a randomized controlled clinical trial.Clin Trials. 2007;4(5):540-7. doi: 10.1177/1740774507083871. Clin Trials. 2007. PMID: 17942469 Clinical Trial.
-
Methods to limit attrition in longitudinal comparative effectiveness trials: lessons from the Lithium Treatment - Moderate dose Use Study (LiTMUS) for bipolar disorder.Clin Trials. 2012 Feb;9(1):94-101. doi: 10.1177/1740774511427324. Epub 2011 Nov 10. Clin Trials. 2012. PMID: 22076437 Free PMC article. Clinical Trial.
-
A New Statistical Method for Designing Stepped-Wedge Cluster Trials [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 May. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2022 May. PMID: 39666843 Free Books & Documents. Review.
Cited by
-
Inverse probability weighting to handle attrition in cohort studies: some guidance and a call for caution.BMC Med Res Methodol. 2022 Feb 16;22(1):45. doi: 10.1186/s12874-022-01533-9. BMC Med Res Methodol. 2022. PMID: 35172753 Free PMC article.
-
Efficacy of e-cigarettes for smoking cessation in populations with psychiatric and/or substance use problems: A secondary analysis of a randomized controlled trial.Tob Prev Cessat. 2025 Feb 3;11. doi: 10.18332/tpc/199473. eCollection 2025. Tob Prev Cessat. 2025. PMID: 39902147 Free PMC article.
-
Protocol of a monocentric, double-blind, randomized, superiority, controlled trial evaluating the effect of in-prison OROS-methylphenidate vs. placebo treatment in detained people with attention-deficit hyperactivity disorder (BATIR).Trials. 2024 Jan 4;25(1):23. doi: 10.1186/s13063-023-07827-7. Trials. 2024. PMID: 38178233 Free PMC article.
-
To attend, or not to attend: Examining caregiver intentions and study compliance in a pediatric, randomized controlled trial.Clin Trials. 2020 Apr;17(2):223-230. doi: 10.1177/1740774519893307. Epub 2020 Jan 27. Clin Trials. 2020. PMID: 31984781 Free PMC article.
References
-
- Leon AC, Demirtas H, Hedeker D. Bias reduction with an adjustment for participants' intent to dropout of a randomized controlled clinical trial. Clinical Trials. 2007;4:540–47. - PubMed
-
- Laird NM. Missing Data in Longitudinal Studies. Statistics in Medicine. 1988;7:305–15. - PubMed
-
- Robins JM, Rotnitzky A, Zhao LP. Analysis of Semiparametric Regression Models for Repeated Outcomes in the Presence of Missing Data. Journal of the American Statistical Association. 1995;90(429):106–21.
-
- Hernán MA, Brumback B, Robins JM. Marginal Structural Models to Estimate the Casual Effect of Zidovudine on the Survival of HIV-Positive Men. Epidemiology. 2000;11(5):561–70. - PubMed
-
- Robins JM, Hernán MA, Brumback B. Marginal Structural Models and Casual Inference in Epidemiology. Epidemiology. 2000;11(5):550–60. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

