Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography
- PMID: 24872543
- PMCID: PMC4067109
- DOI: 10.1093/jnci/dju092
Benefits, harms, and costs for breast cancer screening after US implementation of digital mammography
Abstract
Background: Compared with film, digital mammography has superior sensitivity but lower specificity for women aged 40 to 49 years and women with dense breasts. Digital has replaced film in virtually all US facilities, but overall population health and cost from use of this technology are unclear.
Methods: Using five independent models, we compared digital screening strategies starting at age 40 or 50 years applied annually, biennially, or based on density with biennial film screening from ages 50 to 74 years and with no screening. Common data elements included cancer incidence and test performance, both modified by breast density. Lifetime outcomes included mortality, quality-adjusted life-years, and screening and treatment costs.
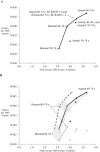
Results: For every 1000 women screened biennially from age 50 to 74 years, switching to digital from film yielded a median within-model improvement of 2 life-years, 0.27 additional deaths averted, 220 additional false-positive results, and $0.35 million more in costs. For an individual woman, this translates to a health gain of 0.73 days. Extending biennial digital screening to women ages 40 to 49 years was cost-effective, although results were sensitive to quality-of-life decrements related to screening and false positives. Targeting annual screening by density yielded similar outcomes to targeting by age. Annual screening approaches could increase costs to $5.26 million per 1000 women, in part because of higher numbers of screens and false positives, and were not efficient or cost-effective.
Conclusions: The transition to digital breast cancer screening in the United States increased total costs for small added health benefits. The value of digital mammography screening among women aged 40 to 49 years depends on women's preferences regarding false positives.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Food and Drug Administration. MSQA National Statistics http://www.fda.gov/Radiation-EmittingProducts/MammographyQuality Standar... Accessed July 1, 2013
-
- Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783 - PubMed
-
- Skaane P. Studies comparing screen-film mammography and full-field digital mammography in breast cancer screening: updated review. Acta Radiol. 2009;50(1):3–14 - PubMed
-
- Vacek PM, Geller BM. A prospective study of breast cancer risk using routine mammographic breast density measurements. Cancer Epidemiol Biomarkers Prev. 2004;13(5):715–722 - PubMed
Publication types
MeSH terms
Grants and funding
- U01 CA152958/CA/NCI NIH HHS/United States
- U01CA69976/CA/NCI NIH HHS/United States
- U01 CA070040/CA/NCI NIH HHS/United States
- U01CA70013/CA/NCI NIH HHS/United States
- U01 CA086082/CA/NCI NIH HHS/United States
- P01 CA154292/CA/NCI NIH HHS/United States
- P01CA154292/CA/NCI NIH HHS/United States
- U01CA63736/CA/NCI NIH HHS/United States
- HHSN261201100031C/CA/NCI NIH HHS/United States
- UC2CA148577/CA/NCI NIH HHS/United States
- U01CA152958/CA/NCI NIH HHS/United States
- UC2 CA148577/CA/NCI NIH HHS/United States
- P30 CA093373/CA/NCI NIH HHS/United States
- U01 CA063740/CA/NCI NIH HHS/United States
- U01CA70040/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- U01CA63740/CA/NCI NIH HHS/United States
- U01 CA063731/CA/NCI NIH HHS/United States
- U01 CA086076/CA/NCI NIH HHS/United States
- U01CA86082/CA/NCI NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- U01 CA069976/CA/NCI NIH HHS/United States
- U01CA86076/CA/NCI NIH HHS/United States
- U01 CA063736/CA/NCI NIH HHS/United States
- U01 CA070013/CA/NCI NIH HHS/United States
- U01CA63731/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

