The timing of dopamine- and noradrenaline-mediated transmission reflects underlying differences in the extent of spillover and pooling
- PMID: 24872568
- PMCID: PMC4035525
- DOI: 10.1523/JNEUROSCI.0166-14.2014
The timing of dopamine- and noradrenaline-mediated transmission reflects underlying differences in the extent of spillover and pooling
Abstract
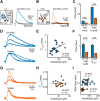
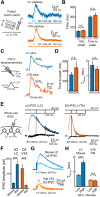
Metabotropic transmission typically occurs through the spillover activation of extrasynaptic receptors. This study examined the mechanisms underlying somatodendritic dopamine and noradrenaline transmission and found that the extent of spillover and pooling varied dramatically between these two transmitters. In the mouse ventral tegmental area, the time course of D2-receptor-mediated IPSCs (D2-IPSCs) was consistent between cells and was unaffected by altering stimulation intensity, probability of release, or the extent of diffusion. Blocking dopamine reuptake with cocaine extended the time course of D2-IPSCs and suggested that transporters strongly limited spillover. As a result, individual release sites contributed independently to the duration of D2-IPSCs. In contrast, increasing the release of noradrenaline in the rat locus ceruleus prolonged the duration of α2-receptor-mediated IPSCs even when reuptake was intact. Spillover and subsequent pooling of noradrenaline activated distal α2-receptors, which prolonged the duration of α2-IPSCs when multiple release sites were activated synchronously. By using the rapid application of agonists onto large macropatches, we determined the concentration profile of agonists underlying the two IPSCs. Incorporating the results into a model simulating extracellular diffusion predicted that the functional range of noradrenaline diffusion was nearly fivefold greater in the locus ceruleus than dopamine in the midbrain. This study demonstrates that catecholamine synapses differentially regulate the extent of spillover and pooling to control the timing of local inhibition and suggests diversity in the roles of uptake and diffusion in governing metabotropic transmission.
Keywords: addiction; integration; monoamine; psychostimulant.
Copyright © 2014 the authors 0270-6474/14/347645-12$15.00/0.
Figures


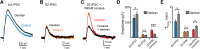


Similar articles
-
Species differences in somatodendritic dopamine transmission determine D2-autoreceptor-mediated inhibition of ventral tegmental area neuron firing.J Neurosci. 2012 Sep 26;32(39):13520-8. doi: 10.1523/JNEUROSCI.2745-12.2012. J Neurosci. 2012. PMID: 23015441 Free PMC article.
-
Mechanisms of 5-HT1A receptor-mediated transmission in dorsal raphe serotonin neurons.J Physiol. 2016 Feb 15;594(4):953-65. doi: 10.1113/JP271716. Epub 2015 Dec 30. J Physiol. 2016. PMID: 26634643 Free PMC article.
-
Noradrenergic Modulation of Dopamine Transmission Evoked by Electrical Stimulation of the Locus Coeruleus in the Rat Brain.ACS Chem Neurosci. 2017 Sep 20;8(9):1913-1924. doi: 10.1021/acschemneuro.7b00078. Epub 2017 Jun 21. ACS Chem Neurosci. 2017. PMID: 28594540
-
[Dopamine receptors and calcium channels regulating striatal inhibitory synaptic transmission].Nihon Yakurigaku Zasshi. 2002 Nov;120(1):61P-63P. Nihon Yakurigaku Zasshi. 2002. PMID: 12491781 Review. Japanese.
-
Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder.J Clin Psychopharmacol. 2008 Jun;28(3 Suppl 2):S46-53. doi: 10.1097/JCP.0b013e318173312f. J Clin Psychopharmacol. 2008. PMID: 18480677 Review.
Cited by
-
Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing.Neuron. 2016 Aug 3;91(3):574-86. doi: 10.1016/j.neuron.2016.06.021. Epub 2016 Jun 30. Neuron. 2016. PMID: 27373830 Free PMC article.
-
Effectiveness and Relationship between Biased and Unbiased Measures of Dopamine Release and Clearance.ACS Chem Neurosci. 2022 May 18;13(10):1534-1548. doi: 10.1021/acschemneuro.2c00033. Epub 2022 Apr 28. ACS Chem Neurosci. 2022. PMID: 35482592 Free PMC article.
-
Properties and modulation of excitatory inputs to the locus coeruleus.J Physiol. 2022 Nov;600(22):4897-4916. doi: 10.1113/JP283605. Epub 2022 Oct 13. J Physiol. 2022. PMID: 36156249 Free PMC article.
-
Relationship between regional gray matter volumes and dopamine D2 receptor and transporter in living human brains.Hum Brain Mapp. 2021 Aug 15;42(12):4048-4058. doi: 10.1002/hbm.25538. Epub 2021 May 20. Hum Brain Mapp. 2021. PMID: 34014611 Free PMC article.
-
Spatiotemporal Control of Noradrenaline-Dependent Synaptic Transmission in Mouse Dorsal Raphe Serotonin Neurons.J Neurosci. 2022 Feb 9;42(6):968-979. doi: 10.1523/JNEUROSCI.1176-21.2021. Epub 2021 Dec 17. J Neurosci. 2022. PMID: 34921047 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
