Orbitofrontal dopamine depletion upregulates caudate dopamine and alters behavior via changes in reinforcement sensitivity
- PMID: 24872570
- PMCID: PMC4035526
- DOI: 10.1523/JNEUROSCI.0718-14.2014
Orbitofrontal dopamine depletion upregulates caudate dopamine and alters behavior via changes in reinforcement sensitivity
Abstract
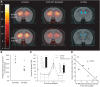
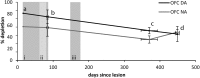
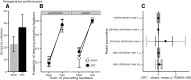
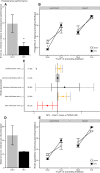
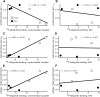
Schizophrenia is associated with upregulation of dopamine (DA) release in the caudate nucleus. The caudate has dense connections with the orbitofrontal cortex (OFC) via the frontostriatal loops, and both areas exhibit pathophysiological change in schizophrenia. Despite evidence that abnormalities in dopaminergic neurotransmission and prefrontal cortex function co-occur in schizophrenia, the influence of OFC DA on caudate DA and reinforcement processing is poorly understood. To test the hypothesis that OFC dopaminergic dysfunction disrupts caudate dopamine function, we selectively depleted dopamine from the OFC of marmoset monkeys and measured striatal extracellular dopamine levels (using microdialysis) and dopamine D2/D3 receptor binding (using positron emission tomography), while modeling reinforcement-related behavior in a discrimination learning paradigm. OFC dopamine depletion caused an increase in tonic dopamine levels in the caudate nucleus and a corresponding reduction in D2/D3 receptor binding. Computational modeling of behavior showed that the lesion increased response exploration, reducing the tendency to persist with a recently chosen response side. This effect is akin to increased response switching previously seen in schizophrenia and was correlated with striatal but not OFC D2/D3 receptor binding. These results demonstrate that OFC dopamine depletion is sufficient to induce striatal hyperdopaminergia and changes in reinforcement learning relevant to schizophrenia.
Keywords: PET; behavior; caudate nucleus; dopamine; orbitofrontal cortex; schizophrenia.
Copyright © 2014 Clarke, Cardinal et al.
Figures
Similar articles
-
Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus.J Neurosci. 2011 Mar 16;31(11):4290-7. doi: 10.1523/JNEUROSCI.5066-10.2011. J Neurosci. 2011. PMID: 21411670 Free PMC article.
-
Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset.Cereb Cortex. 2009 Apr;19(4):889-98. doi: 10.1093/cercor/bhn136. Epub 2008 Aug 22. Cereb Cortex. 2009. PMID: 18723695 Free PMC article.
-
Contrasting effects of excitotoxic lesions of the prefrontal cortex on the behavioural response to D-amphetamine and presynaptic and postsynaptic measures of striatal dopamine function in monkeys.Neuroscience. 1997 Oct;80(3):717-30. doi: 10.1016/s0306-4522(97)00075-4. Neuroscience. 1997. PMID: 9276488
-
Imaging in Parkinson's disease: the role of monoamines in behavior.Biol Psychiatry. 2006 May 15;59(10):908-18. doi: 10.1016/j.biopsych.2005.12.017. Epub 2006 Apr 11. Biol Psychiatry. 2006. PMID: 16581032 Review.
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
Cited by
-
Reversal learning and dopamine: a bayesian perspective.J Neurosci. 2015 Feb 11;35(6):2407-16. doi: 10.1523/JNEUROSCI.1989-14.2015. J Neurosci. 2015. PMID: 25673835 Free PMC article.
-
A framework for the investigation of rare genetic disorders in neuropsychiatry.Nat Med. 2019 Oct;25(10):1477-1487. doi: 10.1038/s41591-019-0581-5. Epub 2019 Sep 23. Nat Med. 2019. PMID: 31548702 Free PMC article. Review.
-
Influence of ventral tegmental area input on cortico-subcortical networks underlying action control and decision making.Hum Brain Mapp. 2018 Feb;39(2):1004-1014. doi: 10.1002/hbm.23899. Epub 2017 Nov 22. Hum Brain Mapp. 2018. PMID: 29165901 Free PMC article.
-
Sex-dependent effects of early life stress on reinforcement learning and limbic cortico-striatal functional connectivity.Neurobiol Stress. 2022 Dec 5;22:100507. doi: 10.1016/j.ynstr.2022.100507. eCollection 2023 Jan. Neurobiol Stress. 2022. PMID: 36505960 Free PMC article.
-
Prefrontal cortex and depression.Neuropsychopharmacology. 2022 Jan;47(1):225-246. doi: 10.1038/s41386-021-01101-7. Epub 2021 Aug 2. Neuropsychopharmacology. 2022. PMID: 34341498 Free PMC article. Review.
References
-
- Bertolino A, Knable MB, Saunders RC, Callicott JH, Kolachana B, Mattay VS, Bachevalier J, Frank JA, Egan M, Weinberger DR. The relationship between dorsolateral prefrontal N-acetylaspartate measures and striatal dopamine activity in schizophrenia. Biol Psychiatry. 1999;45:660–667. doi: 10.1016/S0006-3223(98)00380-1. - DOI - PubMed
-
- Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. doi: 10.1177/0049124104268644. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
