Activation of c-jun N-terminal kinase upon influenza A virus (IAV) infection is independent of pathogen-related receptors but dependent on amino acid sequence variations of IAV NS1
- PMID: 24872593
- PMCID: PMC4136289
- DOI: 10.1128/JVI.00424-14
Activation of c-jun N-terminal kinase upon influenza A virus (IAV) infection is independent of pathogen-related receptors but dependent on amino acid sequence variations of IAV NS1
Abstract
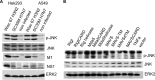
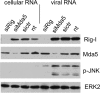
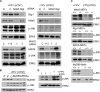
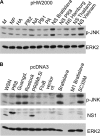
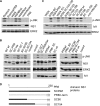
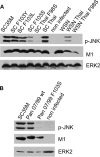
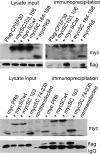
A hallmark cell response to influenza A virus (IAV) infections is the phosphorylation and activation of c-jun N-terminal kinase (JNK). However, so far it is not fully clear which molecules are involved in the activation of JNK upon IAV infection. Here, we report that the transfection of influenza viral-RNA induces JNK in a retinoic acid-inducible gene I (RIG-I)-dependent manner. However, neither RIG-I-like receptors nor MyD88-dependent Toll-like receptors were found to be involved in the activation of JNK upon IAV infection. Viral JNK activation may be blocked by addition of cycloheximide and heat shock protein inhibitors during infection, suggesting that the expression of an IAV-encoded protein is responsible for JNK activation. Indeed, the overexpression of nonstructural protein 1 (NS1) of certain IAV subtypes activated JNK, whereas those of some other subtypes failed to activate JNK. Site-directed mutagenesis experiments using NS1 of the IAV H7N7, H5N1, and H3N2 subtypes identified the amino acid residue phenylalanine (F) at position 103 to be decisive for JNK activation. Cleavage- and polyadenylation-specific factor 30 (CPSF30), whose binding to NS1 is stabilized by the amino acids F103 and M106, is not involved in JNK activation. Conclusively, subtype-specific sequence variations in the IAV NS1 protein result in subtype-specific differences in JNK signaling upon IAV infection.
Importance: Influenza A virus (IAV) infection leads to the activation or modulation of multiple signaling pathways. Here, we demonstrate for the first time that the c-jun N-terminal kinase (JNK), a long-known stress-activated mitogen-activated protein (MAP) kinase, is activated by RIG-I when cells are treated with IAV RNA. However, at the same time, nonstructural protein 1 (NS1) of IAV has an intrinsic JNK-activating property that is dependent on IAV subtype-specific amino acid variations around position 103. Our findings identify two different and independent pathways that result in the activation of JNK in the course of an IAV infection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Quantitative proteomic analysis of the influenza A virus nonstructural proteins NS1 and NS2 during natural cell infection identifies PACT as an NS1 target protein and antiviral host factor.J Virol. 2014 Aug;88(16):9038-48. doi: 10.1128/JVI.00830-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899174 Free PMC article.
-
Influenza A virus NS1 protein-induced JNK activation and apoptosis are not functionally linked.Cell Microbiol. 2017 Jul;19(7). doi: 10.1111/cmi.12721. Epub 2017 Jan 27. Cell Microbiol. 2017. PMID: 28076660
-
Epigenetic Modification Is Regulated by the Interaction of Influenza A Virus Nonstructural Protein 1 with the De Novo DNA Methyltransferase DNMT3B and Subsequent Transport to the Cytoplasm for K48-Linked Polyubiquitination.J Virol. 2019 Mar 21;93(7):e01587-18. doi: 10.1128/JVI.01587-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651365 Free PMC article.
-
The influenza virus NS1 protein as a therapeutic target.Antiviral Res. 2013 Sep;99(3):409-16. doi: 10.1016/j.antiviral.2013.06.005. Epub 2013 Jun 21. Antiviral Res. 2013. PMID: 23796981 Free PMC article. Review.
-
Relevance of signaling molecules for apoptosis induction on influenza A virus replication.Biochem Biophys Res Commun. 2013 Nov 22;441(3):531-7. doi: 10.1016/j.bbrc.2013.10.100. Epub 2013 Oct 28. Biochem Biophys Res Commun. 2013. PMID: 24177013 Free PMC article. Review.
Cited by
-
Role of c-Jun terminal kinase (JNK) activation in influenza A virus-induced autophagy and replication.Virology. 2019 Jan 2;526:1-12. doi: 10.1016/j.virol.2018.09.020. Epub 2018 Oct 10. Virology. 2019. PMID: 30316042 Free PMC article.
-
Cross Talk between MicroRNAs and Dengue Virus.Am J Trop Med Hyg. 2024 Apr 2;110(5):856-867. doi: 10.4269/ajtmh.23-0546. Print 2024 May 1. Am J Trop Med Hyg. 2024. PMID: 38579704 Free PMC article. Review.
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
-
Zoonosis and zooanthroponosis of emerging respiratory viruses.Front Cell Infect Microbiol. 2024 Jan 5;13:1232772. doi: 10.3389/fcimb.2023.1232772. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38249300 Free PMC article. Review.
-
Sperm-Associated Antigen 9 Promotes Influenza A Virus-Induced Cell Death via the c-Jun N-Terminal Kinase Signaling Pathway.mBio. 2022 Jun 28;13(3):e0061522. doi: 10.1128/mbio.00615-22. Epub 2022 May 31. mBio. 2022. PMID: 35638835 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

