Generation of organized germ layers from a single mouse embryonic stem cell
- PMID: 24873804
- PMCID: PMC4050279
- DOI: 10.1038/ncomms5000
Generation of organized germ layers from a single mouse embryonic stem cell
Abstract
Mammalian inner cell mass cells undergo lineage-specific differentiation into germ layers of endoderm, mesoderm and ectoderm during gastrulation. It has been a long-standing challenge in developmental biology to replicate these organized germ layer patterns in culture. Here we present a method of generating organized germ layers from a single mouse embryonic stem cell cultured in a soft fibrin matrix. Spatial organization of germ layers is regulated by cortical tension of the colony, matrix dimensionality and softness, and cell-cell adhesion. Remarkably, anchorage of the embryoid colony from the 3D matrix to collagen-1-coated 2D substrates of ~1 kPa results in self-organization of all three germ layers: ectoderm on the outside layer, mesoderm in the middle and endoderm at the centre of the colony, reminiscent of generalized gastrulating chordate embryos. These results suggest that mechanical forces via cell-matrix and cell-cell interactions are crucial in spatial organization of germ layers during mammalian gastrulation. This new in vitro method could be used to gain insights on the mechanisms responsible for the regulation of germ layer formation.
Figures
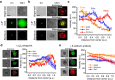



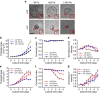

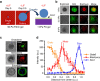

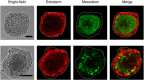
References
-
- Desprat N., Supatto W., Pouille P., Beaurepaire E. & Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470–477 (2008). - PubMed
-
- Rauzi M., Verant P., Lecuit T. & Lenne P. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 10, 1401–U57 (2008). - PubMed
-
- Behrndt M. et al. Forces driving epithelial spreading in zebrafish gastrulation. Science 338, 257–260 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

