The role of antigen specificity in the binding of murine monoclonal anti-DNA antibodies to microparticles from apoptotic cells
- PMID: 24873886
- PMCID: PMC4440675
- DOI: 10.1016/j.clim.2014.05.007
The role of antigen specificity in the binding of murine monoclonal anti-DNA antibodies to microparticles from apoptotic cells
Abstract
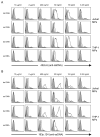
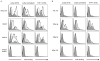
Antibodies to DNA (anti-DNA) are the serological hallmark of systemic lupus erythematosus and markers of underlying immune system disturbances. These antibodies bind to both single-stranded and double-stranded DNA, mediating pathogenesis by forming immune complexes. As shown recently, DNA in blood exists in both free and particulate forms, with DNA representing an important component of microparticles. Microparticles are membrane-bound vesicles containing nuclear molecules, released by membrane blebbing during cell death and activation. A panel of monoclonal NZB/NZW F1 anti-DNA antibodies was tested for binding to microparticles generated from apoptotic THP-1 and Jurkat cells. These studies showed that only certain anti-DNA antibodies in the panel, specific for double-stranded DNA, bound to microparticles. Binding to particles was reduced by soluble DNA or DNase treatment. Together, these results indicate that particle binding is a feature of only certain anti-DNA antibodies, reflecting immunochemical properties of the antibodies and the nature of the exposed DNA antigens.
Keywords: Anti-DNA;; Apoptosis; Autoimmunity;; Lupus;; Microvesicle;.
Copyright © 2014. Published by Elsevier Inc.
Figures



References
-
- Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. - PubMed
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. - PubMed
-
- Mortensen ES, Rekvig OP. Nephritogenic potential of anti-DNA antibodies against necrotic nucleosomes. J Am Soc Nephrol. 2009;20:696–704. - PubMed
-
- van der Vlag J, Berden JH. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol. 2011;31:376–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

